Therapeutics
Latest News
Latest Videos

CME Content
More News

Patients’ near visual needs is important when prescribing medications for glaucoma treatment

Alice T. Epitropoulos, MD, FACS, outlines her approach to managing evaporative dry eye. This includes diagnosing meibomian gland dysfunction, using thermal pulsation to clear gland blockages, and ensuring long-term results with advanced treatments and patient education.

The primary safety endpoint was carried out through the percentage of patients with shift from normal (at baseline) to abnormal in any electrocardiogram (ECG).

The trial is evaluating GAL-101 eye drops in patients with geographic atrophy, an advanced form of dry AMD.

Alon Kahana, MD, PhD, discusses the role of interleukin-6 in thyroid eye disease and the potential of tourmaline Bio's IL-6 inhibitor therapy.
Electrical stimulation therapy is being explored as a potential treatment to enhance neuroplasticity and improve visual function in optic neuropathy patients. Clinical trials show promising results in visual field improvements, though further research is needed on long-term efficacy and quality of life.
According to Iridex Corp., the study confirms MicroPulse TLT's sustained safety and efficacy in managing glaucoma, reducing intraocular pressure and medication reliance with minimal complications over 5 years.

In an interview with Ophthalmology Times, clinical trial investigator Zaina Al-Mohtaseb MD, noted the CLARA Phase 1/2 trial demonstrated the safety, efficacy, and simplicity of corneal endothelial cell injection therapy with a rho kinase inhibitor, offering a less invasive alternative to traditional corneal transplantation for treating corneal edema.
THRIVE-2 met all primary and secondary endpoints at the 15-week primary analysis timepoint after 5 infusions of veligrotug.

According to the company, its Phase 1/2 CLARA trial of AURN001 for corneal edema demonstrated significant dose-dependent efficacy, especially in the high-dose group, with favorable safety and tolerability profiles.
In the LIGHTHOUSE study, Atsena Therapeutics is evaluating ATSN-201 gene therapy for X-linked retinoschisis, leveraging AAV.SPR capsid for central retina transduction without foveal detachment risks.

The 14th annual Glaucoma 360 conference, co-founded by Adrienne Graves, PhD, and Andrew Iwach, MD, and hosted by Glaucoma Research Foundation, will be held from February 6-8, 2025, at the Westin St. Francis in San Francisco, California. The event will focus on the latest advancements in glaucoma care and encourage innovation and collaboration.

Tenpoint Therapeutics and Visus Therapeutics have merged to focus on aging-related ocular conditions, with key products including BRIMOCHOL PF for presbyopia and therapies for cataracts and geographic atrophy.

In an interview with Ophthalmology Times, Alon Kahana, MD, PhD, discusses interleukin-6 and its role in autoimmune diseases, particularly thyroid eye disease, noting that a promising alternative under clinical trial is pacibekitumab, an IL-6 ligand-blocking antibody.

Nona Biosciences has partnered with Kodiak Sciences to develop multi-target antibodies for ophthalmic diseases. Utilizing Nona's Harbour Mice platform, the collaboration aims to accelerate antibody discovery, focusing on innovative therapies for retinal and other eye conditions.

The company begins phase 1 trials of oral GAL-101, targeting amyloid beta aggregation. Promising safety and efficacy in ophthalmic models suggest potential for treating dry AMD, glaucoma, and neurodegenerative eye diseases.
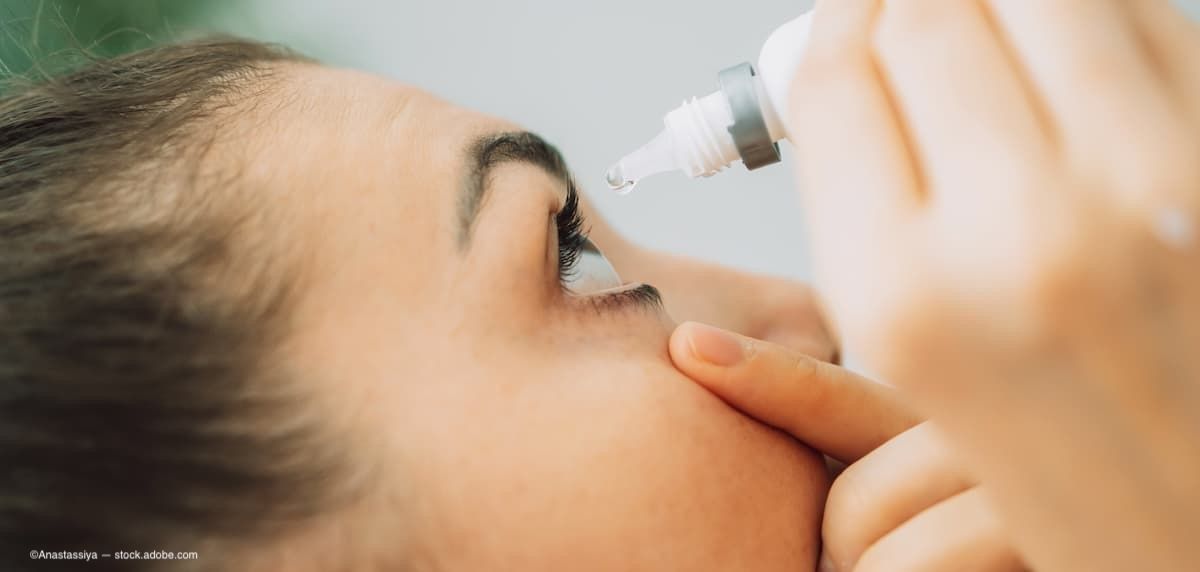
BostonSight’s pilot study, published in Clinical Ophthalmology, explores the use of PROSE scleral lenses as a drug delivery system for cyclosporine 0.05% in treating dry eye disease, showing promising symptom relief and tolerability.

PolyActiva’s phase 2 clinical trial of its PA5108 glaucoma implant met efficacy and safety end points, demonstrating more than 20% reduction in IOP. The biodegradable implant offers sustained drug delivery for up to 26 weeks, addressing challenges of patient adherence and improving glaucoma treatment outcomes.

Melt Pharmaceuticals announced positive results from its Phase 3 study of MELT-300, a non-IV, non-opioid tablet for procedural sedation during cataract surgery. The results support a regulatory submission, with potential to revolutionize sedation practices in various medical specialties.

Emmecell announced positive Phase 1 extension study results for EO2002, a nonsurgical cell therapy for corneal edema. The therapy improved vision, reduced central corneal thickness, and demonstrated strong safety, offering an alternative to corneal transplants.

Retreatment rates are lower, with few orbit decompression procedures.
Livionex, recently announced a successful end-of-Phase 2 meeting with the FDA, supporting the advancement of C-KAD, a 2.6% EDTA ophthalmic solution, to a Phase 3 clinical study. In an interview with Ophthalmology Times, Randall Olson, MD, lead author and a Distinguished Professor at the University of Utah, Chair of the Department of Ophthalmology and Visual Sciences, and CEO of the John A. Moran Eye Center, discusses the ongoing research.

Sun Pharmaceutical presented Phase 4 data at ESCRS 2024 showing that cyclosporine ophthalmic solution 0.09% significantly improves dry eye disease symptoms and corneal staining in patients inadequately controlled on Restasis, with positive results observed through 12 weeks of treatment.