Technology
Latest News

Latest Videos

CME Content
More News

The Iridex PASCAL combines industry-leading speed and precision pattern scanning, Endpoint Management Technology, Pattern Scanning Laser Trabeculoplasty, and now, MicroPulse Technology, in an integrated laser platform using half the footprint of its predecessor.

Physicians are using the procedure to treat various stages of the disease.

According to the company, the outcomes reported demonstrate a favorable safety profile for its novel, noninvasive glaucoma treatment.

AVA-Net is a deep-learning network developed by scientists from the University of Illinois at Chicago.

The Centre for Ocular Research and Education is demonstrating its latest discoveries for eye applications at the Association for Research in Vision and Ophthalmology’s annual meeting in New Orleans.

As technology advances, innovations increase physician capabilities.
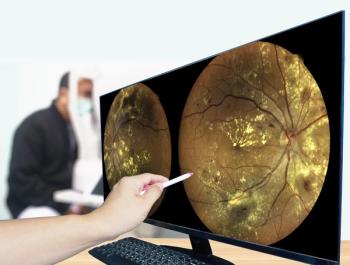
The technology could prove to be the foundation for establishing a new biomarker of ocular diseases.

SeeLuma is a “Fully Digital Surgical Visualization Platform” to assist ophthalmic surgeons.

Eyenuk Inc. founder and CEO Kaushal Solanki, PhD, went from working for the Defense Department to founding a company with a focus on artificial intelligence for ophthalmology. Find out what led him to make that jump.

SMSGOP and SMGOP are two models of artificial intelligence that can predict progression of Graves’ orbitopathy.

Technology offers accessibility, scalability to countries where care is needed the most.
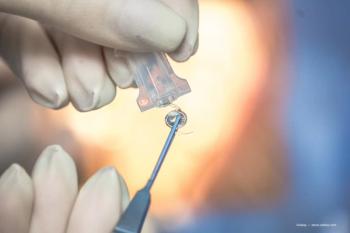
Evolving technology is resulting in better outcomes for patients.
Nitish Mehta, MD discusses the latest trends in Artificial Intelligence and Machine Learning technology in retinal imaging and its uses for ophthalmologists.

Physician discusses preoperative assessments before procedure focusing on cornea.

The new laser will allow surgeons to perform refractive correction on patients with myopia, with or without astigmatism using the SILK Procedure.

Artificial intelligence (AI) with ultra-widefield fundus autofluorescence images may be helpful to objectively estimate progression of retinitis pigmentosa (RP) and visual function.

Having the best tools available is of the utmost importance in the operating room (OR).

According to the company, the app features new content, including podcasts, discussion forums and downloadable content for eyecare professionals.

Physician discusses existing advancements, innovations for treatment.

Bausch + Lomb and Modulight announce the photodynamic laser for use with photodynamic therapy was approved by the FDA for the treatment of patients with subfoveal choroidal neovascularization due to AMD.

With donations, the West Virginia University Department of Ophthalmology and Visual Sciences has added two new pieces of ophthalmic training equipment to its lab.

Researchers are looking for improved detection of disease in patients.

Runa Capital-led funding will help scale the AI-powered eye pathology detection solution.
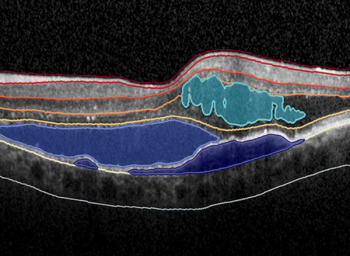
Discovery CORE and its AI models have been designed to accelerate data analysis and help clinical and academic researchers collaborate more efficiently in real time with their peers on medical and imaging datasets.

In a two-decade career as a researcher at Johnson & Johnson Surgical Vision, Piers has been a part of teams at the forefront of a number of innovations in IOLs. She discusses her career and the outlook for research and development in ophthalmology.