Specialists seek more information for use in the treatment of patients diagnosed with retinal diseases


Specialists seek more information for use in the treatment of patients diagnosed with retinal diseases

HG004 aims to be more effective than other AAV2 products in treatment of inherited retinal diseases caused by mutations in the RPE65 gene.

The workshop is designed to bring together leading experts from academia and industry, to discuss openly, and in detail, what is known about PRPH2 disease pathology, disease models, clinical characteristics, and therapeutic approaches.

Patients diagnosed with diabetic retinopathy may be particularly vulnerable to periods of low glucose, and keeping glucose levels stable should be an important part of glucose control.

Carolyn Majcher, OD, FAAO, provides an overview of the symptoms of diabetic retinopathy and diabetic macular edema (DR/DME) and recommendations for dilated eye exams for patients with type 1 and type 2 diabetes.

Carolyn Majcher, OD, FAAO, and Rishi Singh, MD, discuss the current prevalence and future projections of diabetic retinopathy and diabetic macular edema (DR/DME) as well as the impact of early diagnosis and treatment for patients with diabetic retinal diseases.

Let us know how familiar are you on the approval process for biosimilar drugs compared with new drug applications to the FDA. The poll will be open until February 28, 2023.

According to the company, it is planning to hold an end-of-phase 2 meeting with the FDA to discuss the results.
A team of researchers have found that STOC tomography enables unprecedented views of eye structure.

Researchers used obesity as a model to accelerate and exaggerate the stressors experienced by the body throughout life.

The study advances the understanding of the leading cause of visual impairment in adults.

Researchers developed nanoparticles able to penetrate the neural retina and deliver mRNA to the photoreceptor cells whose proper function makes vision possible.
Investigators found that all patients had similar single surgery success rates but that minority patients had significantly worse vision outcomes and were more likely to have multiple retinal breaks.

In a conversation with David Hutton of Ophthalmology Times®, Ferhina S. Ali, MD, MPH, discussed the real world use of faricimab in the IRIS Registry during a presentation this week at Hawaiian Eye in Kauai Hawaii, focusing on the use of faricimab for the treatment of neovascular AMD and diabetic macular edema.
According to researchers, factors that were associated with all type of visual impairment included older age, lower education level, and lower income were associated with all types of visual impairment.

Diabetic retinopathy is the leading cause of blindness in American adults, and a team of researchers believes the source of this damage may lie in the belly — mainly a leaky small intestine. A novel treatment can possibly prevent or reverse this damage.

Continued education and financial incentives will become essential if biosimilars are to become a mainstay in ophthalmological markets, according to a recent report.
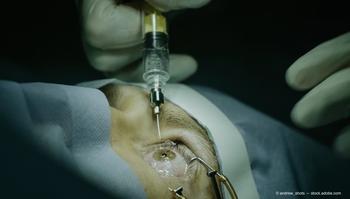
The researchers conducted a retrospective chart review of patients with typical exudative AMD that had been treated with anti-VEGF therapy injections.


FT-001 is administered by a one-time injection into the subretinal space of the eye that delivers a functional copy of the human RPE65 gene to the nuclei of the patient’s retinal cells.
The delivery of VivaVision's molecules via the suprachoroidal space using Everads' technology is expected to lead to novel treatments for retinal diseases.
A team of researchers from the University of Wisconsin–Madison developed a way to grow organized clusters of cells, called organoids, that resemble the retina, the light-sensitive tissue at the back of the eye.

According to a news release, Opus will advance preclinical development programs for BEST1– and RHO-related retinal diseases. The deal expands the company’s addressable patient population for its novel treatments for rare inherited retinal diseases.

According to investigators, the study demonstrated that by restoring the function of ADAM10, a major shedding protein, it was possible in preclinical models to control the abnormal formation of blood vessels, offering an attractive therapeutic target to treat DR.
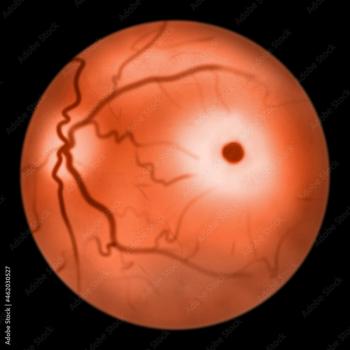
The National Institute on Aging of the National Institutes of Health (NIH) is expanding the effort to understand that connection with a $10.3 million grant.