
Eye rubbing is a common practice, but one that can cause a number of problems.


Eye rubbing is a common practice, but one that can cause a number of problems.

Alice Epitropoulos, MD, goes over some tips and methods to educate patients about OSD before performing any surgery.

Patients treated with azithromycin did not have a higher incidence of gastrointestinal adverse events.
Outcomes achieved with information, osmolarity testing, proven treatments
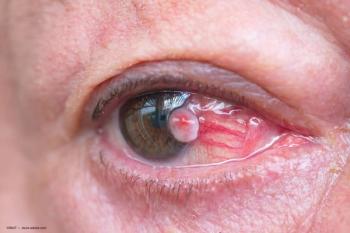
Ophthalmologists should be aware of a patient’s prior history

Jeff Nau, PhD, MMS, President and CEO of Oyster Point Pharmaceuticals, provides an appraisal of the company's pipeline as well as updates on recent innovations to hit the market.

Those who used both traditional and electronic cigarettes reported severe to very severe ophthalmic symptoms.

Patients are at risk for developing severe dry eye and ocular surface disease.

During a presentation at the Association for Research in Vision and Ophthalmology’s 2022 annual meeting in Denver, Yuichi Hori, MD, PhD, and colleagues found that taping the top border of a surgical mask to a clinicians’ skin reduces the potential for ocular surface damage resulting from expirations of air reaching the ocular surface during the COVID-19 pandemic.
Severe ocular involvement demands inflammation control

A team of investigators from Pohang University of Science and Technology has found that conjunctival goblet cell examination is important for the precise diagnosis and effective treatment of ocular surface diseases; however, CGC examination has not been possible until now due to lack of non-invasive devices.

Cynthia Matossian, MD, shares her thoughts on the premium patient "experience" or "journey," emphasizing the outcomes of patient experiences.

Dongkyun Lang, a biomedical engineering and optical sciences professor, is developing a way to diagnose and treat corneal ulcers that is eight times cheaper and 20 times faster than today's gold standard.

Cynthia Matossian, MD, FACS, ABES, points out that there has been confusing news lately about what is (and is not) covered by Medicare when it comes to in-office treatments for meibomian gland dysfunction.
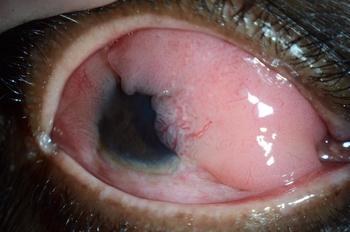
Nathan Hall, BS, MS, presents research on how epidemiologic analysis of malignant ocular surface tumors found significant differences in geographic prevalence rates in the United States.
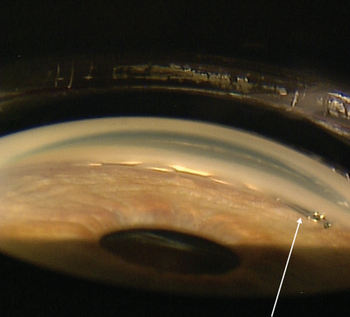
Three cases demonstrate value of new tools, technology for surgeons.

According to investigators, aqueous humor analysis differentiated the tumors in patients.

Investigators find that long-term ocular complications require further observation.

Cynthia Matossian, MD, FACS, ABES, takes a look at the recent, sudden change in low-payment reimbursement by Medicare Administrative Contractors for LipFlow and MGD procedure codes.

Symptomatic, psychosocial burdens in participants .

CAM’s regenerative healing means long-term relief and fewer visits to clinic.
Tool for quantifying measures may predict outcomes for patients.

According to Oculus, its Myopia Master saves space and can be mounted on a workstation or an ophthalmic table. The software is operated directly via an inbuilt display.

The company is evaluating topical cyclosporine to treat the signs and symptoms of dry eye disease.
According to the Refractive Surgery Council, patients continue to have a high level of interest in laser vision correction procedures.