
Renewed consumer interest in and demand for LASIK, SMILE, and other procedures are among the key drivers for 2021 performance.

Renewed consumer interest in and demand for LASIK, SMILE, and other procedures are among the key drivers for 2021 performance.
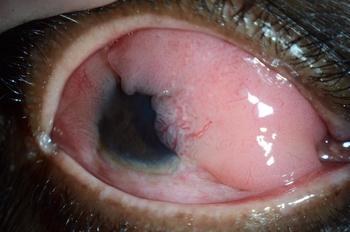
Investigators find the prevalence of ocular surface tumors is greater in the North Central region of the United States.

According to the companies, the agreement will open a channel between federal health facilities and CorneaGen's laboratories.
According to the company, A197 is a novel, first-in-class, topical immunomodulatory agent which will move into Phase II clinical trials in dry eye patients.
The application of mitomycin C after corneal cross-linking does not prevent development of corneal haze after the procedure, but it does contribute to development of more corneal haze.

Delayed presentations and acute shortage of donor corneal tissues for emergency keratoplasty are among reasons given for adverse outcome.

With every 10-year increase in patient age, the risk of the patients needing concomitant administration of steroid eye drops decreased by half, according to investigators.

A team of researchers from Thomas Jefferson University have found that immune cells could be doing much more than first thought in protecting our eyes.

Analysis finds acceptable outcomes can be expected when pterygium surgery is performed by a supervised ophthalmology resident.

New cell therapy drug makes treatment of corneal endothelial disease more accessible.

According to the organization, the goal is to highlight and address the diverse and critical vision and eye health needs of children and to improve outcomes through advocacy, public health, education, and awareness.

Although graft rejection has not been associated with vaccinations, concern remains.
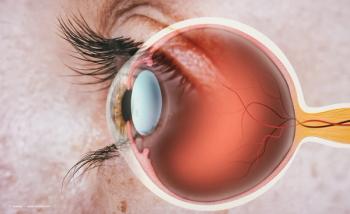
Impairment of corneal sensory innervation causes reduction of both protective reflexes and trophic neuromodulators that are essential for the vitality, metabolism, and wound healing of the ocular surface.

According to Lisa Nijm, MD, JD, patients can benefit from short-term, on-label treatment with a novel loteprednol formulation

I-MED Pharma Inc., a Canadian-based firm, announced this week the expansion of its strategic partnership with ESW Vision in France to be their exclusive distributor in the United States.

TearLab Corp. announced that Adam Szaronos has been appointed president and CEO by the company's Board of Directors, effective immediately.

Investigators are hopeful that future research can focus on public health concerns of dry eye disease.
The company will begin commercialization of the IC-8 small aperture IOL, used for cataract patients, upon successful completion of the manufacturing facility inspections and receipt of an official approval order from the FDA, which the company estimates in Q2 2022.

Study shows statistically significant improvement for primary endpoint of bulbar conjunctival hyperemia for OTX-DED 0.2 mg and 0.3 mg formulations compared with vehicle hydrogel insert.

Successful treatment options can offer hope for patients with inherited disease.

Approaches include anti-vascular endothelial growth factor agents, fine needle diathermy, and corneal collagen crosslinking.
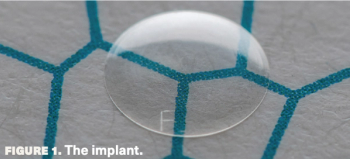
New acrylic implant provides option for patients with endothelial dysfunction.
Shady Awwad, MD, discusses how the application of mitomycin C after corneal crosslinking does not prevent development of corneal haze after the procedure and actually contributes to development of more corneal haze.

Procedure transfers intact donor nerve fibers to the cornea.
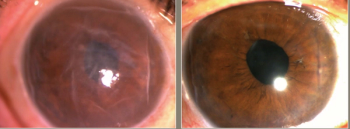
Results from Aurion Biotech's IOTA trial reveals that a corneal endothelial cell therapy showed improvements in visual acuity and central corneal thickness, according to a team of Japanese investigators.