Ora Chief Medical Officer Gustavo De Moraes, MD, PhD, MPH, speak with Ophthalmology Times®' Sheryl Stevenson to discuss some of the cutting-edge advancements in therapies and diagnostics for the treatment of glaucoma patients.

Ora Chief Medical Officer Gustavo De Moraes, MD, PhD, MPH, speak with Ophthalmology Times®' Sheryl Stevenson to discuss some of the cutting-edge advancements in therapies and diagnostics for the treatment of glaucoma patients.

Glaucoma 360 is being held at the Grand Hyatt San Francisco at Union Square, and the meeting brings together celebration, innovation, and education in its efforts to eradicate glaucoma, with the Gala serving as the traditional kickoff of the event.
Glaucoma 360's co-founders and co-chairs Adrienne L. Graves, PhD, and Andrew G. Iwach, MD, speak with Ophthalmology Times®' Sheryl Stevenson on what attendees can expect from this year's annual meeting.
A University of Montréal Hospital Research Center team reveals the fine mechanisms behind the major vascular defects observed in glaucoma patients and identifies new therapeutic targets.

According to the company, its C.STIM IPL system is based on intense pulsed light technology for the treatment of dry eye disease.

The Toronto-based medical eyewear company partnered with the visual assistance community to help empower an Oregon college student with enhanced vision.

Presbyopia-correcting drops represent a whole new product category—one with a lot of upsides for clinicians who want to help their patients see well at all distances.
Moore suffered from dry eye disease and Lumenis noted that treatment with OptiLight improved her condition. Now she’s partnering with the company to share her story and empower others.

The company completed enrollment ahead of schedule in a second Phase 3 FDA registration trial for Nyxol in RM with top-line results expected by the end of the first quarter.

The latest invention from Mark S. Humayun, MD, PhD, brings hope to sufferers of age-related macular degeneration, a common type of blindness.
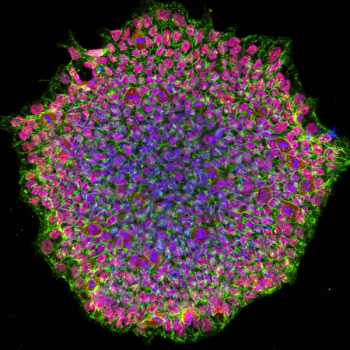
The team hopes the use of patient-derived stem cells will enable high-throughput drug screening for potential therapeutics for patients.
The company noted that the study was the first to evaluate whether AI software can accurately detect more-than-mild diabetic retinopathy using a single image per eye, obtained from either a desktop or handheld retinal camera.

The 3-month study will assess the safety and ocular hypotensive efficacy of TC-002 ophthalmic solution.

The FDA has approved the first generic of cyclosporine ophthalmic emulsion (Restasis; Allergan) 0.05% single-use vials of eye drops to increase tear production in patients with dry eye disease.
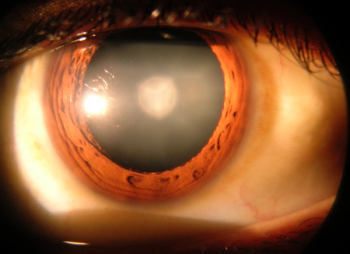
Researchers at the University of Arizona Health Sciences will use a $1.6 million National Eye Institute grant to study age-related lens cell changes that can lead to cataracts.
According to investigators, the implant can provide a sustained infusion of drug to the retina

A team of investigators has conducted ocular pharmacokinetic studies, including metabolism, providing valuable information for ocular drug development.

A team of investigators examined the association between COVID-19 transmissibility and elapsed time since infection, and found that SARS-CoV-2 may still be transmissible 10 days post-infection.

Cleveland Eye Bank Foundation (CEBF) will feature nine researchers from three eye institutions on February 15, from 3 to 5 pm Eastern, for its second annual virtual vision research symposium.

The future of this age-related condition is bright and in focus.

A new treatment option may increase basal tear production in patients.

Faricimab is the first and only FDA-approved medicine targeting two distinct pathways, Ang-2 and VEGF-A, that often cause retinal diseases that may cause vision loss.

Joshua Mali, MD, shares how the FDA-approved faricimab-svoa (Vabysmo, Genentech) will change the treatment landscape for wet AMD and DME.

The study reviews the outcomes following penetrating, closed trauma–related injuries.

The historic eye institute celebrates six decades of leadership in eye care research, education and clinical practice.