A team of investigators from the University of Michigan may have unlocked a potential new recipe for counteracting the impact that COVID-19 has on patients with underlying disease processes such as type 2 diabetes.

A team of investigators from the University of Michigan may have unlocked a potential new recipe for counteracting the impact that COVID-19 has on patients with underlying disease processes such as type 2 diabetes.
The study investigated rates of cognitive impairment among survivors of COVID-19 who had been treated in outpatient, emergency department, or inpatient hospital settings.

The Academy's annual meeting set for November 12 to 15 in New Orleans.
According to investigators, the risk of death from strokes, heart attacks and other vascular causes is increased by more than a third.

According to a team of French investigators, every patient hospitalized with severe COVID-19 cases in their study had retinal and choroidal anomalies on indocyanine green angiography and optical coherence tomography images that were possibly related to the virus.

Bausch + Lomb and Clearside Biomedical announced Monday that the U.S. Food and Drug Administration has approved XIPERE (triamcinolone acetonide injectable suspension) for suprachoroidal use for the treatment of macular edema associated with uveitis.

According to the company, Susvimo is a first-of-its-kind therapeutic approach for wet AMD and may help people with the disease maintain their vision with as few as two treatments per year.

A coalition of vision groups held a briefing on Capitol Hill this week to educate legislators on increasing incidence of myopia and the associated cost burden.

Fellowship recipient Christopher J. Kaler will explore functional links between BAP1 loss and aberrant activity of the nucleosome remodeling and deacetylase complex, which normally mediates embryonic lineage commitment and cellular differentiation.

The Delta variant still predominates in the US and UK, but the CDC mentions the AY variants as a concern, and suggested that it may be more contagious and more severe than the original COVID-19 strain.
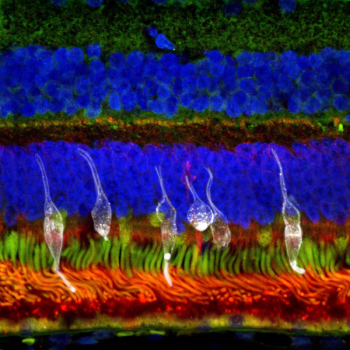
In a study, investigators demonstrated the safety and therapeutic potential of clinically compliant iPSC-derived photoreceptor precursors as a cell replacement source for future clinical trials.

Oyster Point Pharma, Inc. announced Monday that the U.S. FDA has granted approval of its TYRVAYA (varenicline solution) nasal spray 0.03 mg for the treatment of dry eye disease (DED), making it the first — and only — nasal spray approved for DED treatment in the U.S

Longtime clinician, researcher and head of the Ophthalmology Department at UCSF will succeed David W. Parke II, MD, at Academy helm.
The FDA will decide whether to authorize the boosters based off the advisory panel's recommendations.
Ocular herpes infection is a leading cause of infectious blindness and can lead to lethal brain infections. Using genetically modified mice models, investigators uncovered the importance of mTORC2 in activation of innate- and virus-adaptive immunity during ocular HSV-1 infection.

To mark World Sight Day, Nanodropper Inc. has developed a pay-it-forward Nanodropper Adaptor program, allowing anyone to donate devices to a pool for distribution to patients in need.

As the world comes together to shine a global spotlight on blindness and vision impairment on World Sight Day, several global organizations and companies are stepping up to increase awareness of eye health, including the importance of regular examinations by an ophthalmologist or optometrist.
Oxurion NV announced Wednesday the first patient to be dosed in the INTEGRAL phase 2 clinical study evaluating THR-687 as a treatment for patients with DME.

Cynthia Matossian, MD, FACS, ABES, takes a look at the recent, sudden change in low-payment reimbursement by Medicare Administrative Contractors for LipFlow and MGD procedure codes.

A simple solution that involves use of air filtration devices may help reduce the risk of hospital-acquired SARS-CoV-2 infections.

Aerie Pharmaceuticals announced Tuesday positive topline results for the phase 3 trial evaluating netarsudil 0.02% versus ripasudil 0.4% for the treatment of open-angle glaucoma and elevated IOP.

The FDA approved Ocular Therapeutix Inc.’s Supplemental New Drug Application (sNDA) that further extends Dextenza’s (dexamethasone ophthalmic insert) 0.4 mg indications, the company announced Monday.
Investigators in the United Kingdom found that concomitant vaccination of flu and COVID-19 raised no safety concerns and preserved the immune response to both vaccines.

According to 4D Molecular Therapeutics, it has received FDA Clearance of an IND Application for 4D-150, a dual-transgene intravitreal gene therapy for patients with wet AMD.
The company will move the highest dose of THR-149 (0.13mg) into Part B of the study based on favorable safety profile and positive efficacy data.
iCare USA’s EIDON Ultra-Widefield Lens module has received FDA 510 (k) approval for distribution across the United States, the company announced Wednesday.

The American Society of Retina Specialists (ASRS) will host its 39th Annual Scientific Meeting from October 8-12, 2021, at the JW Marriott San Antonio Hill Country Resort and Spa in San Antonio, Texas.
Investigators found evidence of improvement in vision and retinal structure in patients with DME and AMD sustained through 12 weeks.

A team of investigators at the Texas Tech University Health Sciences Center have developed a technology derived from corneal epithelial stem cells to improve outcomes for DED patients.

According to researchers at the University of Virginia School of Medicine, common HIV drugs or a safer alternative could stop vision loss.