Researchers at the University of Bristol, as part of a 10-year follow-up study, found that severe “brain bleeds” experienced by some babies in the first year following their birth lead to long-term sight problems.

Researchers at the University of Bristol, as part of a 10-year follow-up study, found that severe “brain bleeds” experienced by some babies in the first year following their birth lead to long-term sight problems.

Kiora Pharmaceuticals announced it has enrolled the first patient as a part of a Phase 2 study evaluating KIO-201 topical eye drops in patients with PCED, a rare ocular surface condition characterized by non-healing wounds on the eye surface.

A team of investigators at the Agency for Science, Technology and Research in Singapore have developed a bio-functional thermogel, a type of synthetic polymer, to prevent retinal scarring caused by failed retinal detachment repair surgery.
Using a cloud-based digital health option can save time during surgery.

A new deep learning model that can identify disease-related features from images of eyes has been unveiled by a group of investigators from Tohoku University.
ASCRS, AAO behind year-long drive for policy change, with Aetna dropping its requirement on July 1 for pre-authorization for cataract surgery, covering patients in 48 of 50 states.
David Brown, MD, highlights his ASRS poster that includes 44-week data for a four-times-higher dose of aflibercept in a real-world setting.
Rahul Khurana, MD, shares a preview of his ASRS poster based on an analysis of the second year of the VIEW 1 and VIEW 2 studies comparing aflibercept and ranibizumab treatment arms.
Investigators have found that recordings from the retina could identify distinct signals for both attention deficit hyperactivity disorder and autism spectrum disorder, providing a potential biomarker for each condition.

A team of investigators from the Yong Loo Lin School of Medicine at the National University of Singapore has found that there is a bidirectional association between chronic kidney disease and glaucoma.

RegeneRx Biopharmaceuticals Inc. said its U.S. joint venture partner and licensee, HLB Therapeutics, will expand its ophthalmic clinical development program with RGN-259 in two indications, neurotrophic keratopathy and dry eye disease.

A team of investigators at Flinders University in Australia have found that a specific cell within the retina appears to be particularly good at housing Ebola and other viruses.

As ophthalmologists observe Cataract Awareness Month this June, William L. Soscia, MD, medical director at Center for Sight, in Sarasota, Florida, focuses on the importance of having excellent vision for safety and everyday functions.

The U.S. Centers for Medicare and Medicaid Services code is effective July 1 and will enhance access to Xipere, the only therapy available in the United States for suprachoroidal use in the treatment of macular edema associated with uveitis.
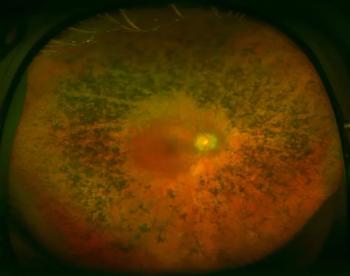
A team led by researchers from the University of Geneva has identified a molecular mechanism that causes degeneration of the eye’s photoreceptors, which can lead to blindness.

Jason Menzo is named CEO, while Russell Kelley, PhD, MBA, will become managing director of the RD Fund. Ben Yerxa, PhD, will take the helm of Opus Genetics, the first RD Fund- and foundation-backed spin-off company.

Researchers believe this may be the first report of the use of topical brimonidine gel for the treatment of eyelid ptosis associated with cosmetic use of botulinum toxin.

A study by Orbis International found that children with myopia experienced significantly higher levels of depression and anxiety than their peers without vision impairment.
New lens types offer independence from glasses to the patient post surgery.

Foundation Fighting Blindness is among a group of investors offering financial support for Nacuity’s efforts to stop oxidative tissue damage, a driver of blinding eye diseases.
According to the organization, the eBook content is designed for residents, ophthalmologists, fellows, medical students and other professionals performing or supporting cataract surgery.
As dexamethasone adds indications, a study confirms efficacy of punctal occlusion for allergic conjunctivitis.

The treatments typically include application of heat, lid hygiene, and massage of eyelid.
Having a comprehensive team of ophthalmic specialists in place means patients could have multiple issues treated in a single procedure that might require two surgeries otherwise.


Ophthalmic World Leaders (OWL) will be hosting an OWL Connect event during the American-European Congress of Ophthalmic Surgery (AECOS) 2022 Summer Symposium, Saturday, July 9.

The study investigates 3-year intravitreal implant in eyes with active noninfectious posterior uveitis.
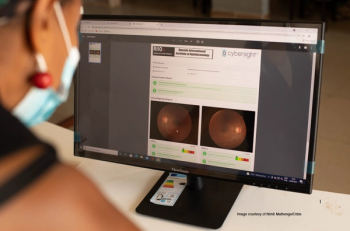
The research from eye care nonprofit Orbis International focuses on diabetic retinopathy in Rwanda, where associated vision complications from diabetes are growing.

The systems could be in the hands of surgeons in the third quarter through a targeted initial launch, according to the company.