
Procedure transfers intact donor nerve fibers to the cornea.

Procedure transfers intact donor nerve fibers to the cornea.

Targeted antibody testing and therapy offers new options for ophthalmologists
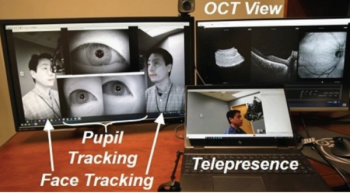
Pilot study tests the technology to obtain remote fundus imaging of patients.

Investigators find that mutations could lead to early treatment intervention.
The FDA has given its approval to Byooviz (ranibizumab-nuna, SB11, Samsung Bioepis Co Inc and Biogen Inc), a biosimilar referencing Lucentis (ranibizumab, Genentech).
According to investigators, the SARS-CoV-2 spike protein physically interacts with GRP78 on the cell surface in patients with diabetes and promotes the binding to and accumulation in angiotensin-converting enzyme 2 (ACE2)-expressing cells.
Ongoing nationwide study looks at in vitro antibiotic resistance among isolates.
Sustained-release option is deposited directly into the lenticular capsule.
A team of investigators from the University of Michigan may have unlocked a potential new recipe for counteracting the impact that COVID-19 has on patients with underlying disease processes such as type 2 diabetes.

Patients can get real-time disease monitoring with self-operated device.
The study investigated rates of cognitive impairment among survivors of COVID-19 who had been treated in outpatient, emergency department, or inpatient hospital settings.

Improvements are seen after gene therapy for Leber congenital amaurosis.

According to a team of French investigators, every patient hospitalized with severe COVID-19 cases in their study had retinal and choroidal anomalies on indocyanine green angiography and optical coherence tomography images that were possibly related to the virus.
Investigators have found that the early and late stages of uveal melanoma differ markedly.

All of the newborns in a recent study had ocular manifestations, the most common being periorbital edema and hemorrhagic conjunctivitis,
The development of involutional ptosis may be a multifactorial process, researchers have found.

According to investigators, aqueous humor analysis differentiated the tumors in patients.

The Delta variant still predominates in the US and UK, but the CDC mentions the AY variants as a concern, and suggested that it may be more contagious and more severe than the original COVID-19 strain.

Demographics show high demand for solutions beyond eyeglasses.
A team of Israeli investigators sought to characterize breakthrough cases by evaluating health care workers with COVID-19 symptoms or who had been exposed to the virus.
New data finds that preoperative ptosis occurs commonly and more often in patients with reactive blepharospasm or enophthalmos.
The FDA will decide whether to authorize the boosters based off the advisory panel's recommendations.
A team of investigators at Cleveland Clinic Abu Dhabi studied patients who presented with ocular adverse events within 15 days after the first of 2 doses of the inactivated COVID-19 vaccine Sinopharm.

Investigators find that long-term ocular complications require further observation.

To mark World Sight Day, Nanodropper Inc. has developed a pay-it-forward Nanodropper Adaptor program, allowing anyone to donate devices to a pool for distribution to patients in need.

A simple solution that involves use of air filtration devices may help reduce the risk of hospital-acquired SARS-CoV-2 infections.

The FDA approved Ocular Therapeutix Inc.’s Supplemental New Drug Application (sNDA) that further extends Dextenza’s (dexamethasone ophthalmic insert) 0.4 mg indications, the company announced Monday.
Merck and Ridgeback Biotherapeutics are seeking emergency use authorization for molnupiravir, an oral experimental antiviral treatment for mild-to-moderate COVID-19 in adults at risk for virus progression or hospitalization.
David Boyer, MD, reports on the use of pegcetacoplan to treat geographic atrophy when administered monthly or every-other-month regimens in the phase 3 DERBY and OAKS studies.

Arshad Khanani, MD, MA, reports primary study findings from the phase 3 study on the Port Delivery System with ranibizumab.