The study focused the safety and tolerability of increasing doses of polihexanide eye drops in order to choose a dose that would be tested in a phase III clinical trial in patients with acanthamoeba keratitis.

The study focused the safety and tolerability of increasing doses of polihexanide eye drops in order to choose a dose that would be tested in a phase III clinical trial in patients with acanthamoeba keratitis.
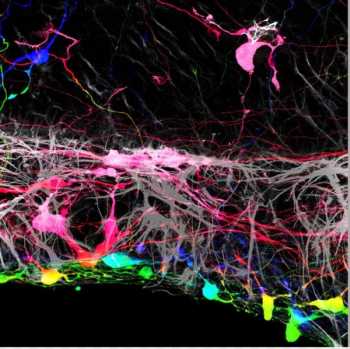
Investigators at the Wilmer Eye Institute at the Johns Hopkins University School of Medicine conducting experiments in mouse tissues and human cells found that they may be able improve the success rate for regrowing nerve cells damaged by blinding diseases.

The Phase 1 clinical trial of EyePoint Pharmaceuticals Inc.’s EYP-1901 is underway. It is a potential twice-yearly sustained delivery anti-VEGF treatment targeting wet age-related macular degeneration.
Visus Therapeutics CEO Ben Bergo shares an update on the status of its lead product candidate for presbyopia treatment as well as what's in the company's pipeline for 2021.

The FDA has granted Fast Track designation to MeiraGTx's AAV-CNGA3 gene therapy product candidate for the treatment of achromatopsia (ACHM).

Ocular symptoms in COVID-19 patients may be more common than previously thought — with sore eyes a significant sign of disease.

In observance of International Holocaust Remembrance Day this January 27, John H. Merey, MD, may be the only Holocaust survivor still in the practice of ophthalmology.

The investigational therapy met all primary endpoints of absence of inflammation at both Day 8 and Day 15 for the treatment of pain and inflammation following cataract surgery.

Neurophth Therapeutics, Inc (Neurophth) and AAVnerGene Inc have announced the launch of a strategic partnership that will grant Neurophth global rights to mutually select adeno-associated virus (AAV) capsids for the creation of the next-generation ophthalmic gene therapy.

Bausch + Lomb has enrolled 599 participants in the first of two Phase 3 studies evaluating perfluorohexyloctane as a first-in-class investigational drug to treat the signs and symptoms of dry eye disease associated with meibomian gland dysfunction.

A device developed by an Israeli startup was implanted in the eye of a patient, enabling him to recognize his family and read words.

See what ranked most popular in our top 10 stories of 2020.

Amid ramped up production of vaccines, CARES Act gives FDA power to head off potential drug shortfalls. Prevent Blindness is urging the FDA to use its authority to ensure TED treatment drug supply isn’t interrupted.

Delivery into the suprachoroidal space may potentially extend the duration of therapeutic action and reduce or relieve the treatment burden for wet AMD.

Amydis announced the successful completion of a pre-investigational new drug (IND) meeting with the FDA for its candidate AMDX-2011P, a small-molecule retinal tracer that targets amyloid beta to diagnosis amyloid angiopathy.

Two teams of researchers from Gyroscope Therapeutics and the University of Pennsylvania are joining forces to explore gene therapy targets for three specific serious eye diseases.

Tamara R. Fountain, MD, will serve as president of the American Academy of Ophthalmology after being elected by the organization’s community of ophthalmologists during its recent virtual annual meeting.
Topline data following completion of ALTISSIMO trial treatment phase expected in second quarter of 2021.

Roche’s faricimab is the focus of Yosemite and Rhine studies, which investigators say show it has the potential to offer lasting vision improvements for patients with diabetic macular edema.

The platform can autonomously diagnose vision defects and customize individual vision augmentation based on the user’s vision defects.

In light of an unprecedented year filled with technological advancements and pivots, Joshua Mali, MD, offers his top 5 predictions in ophthalmology for 2021.
Joshua Mali, MD, speaks on his annual top 5 predictions in ophthalmology for what's to come in 2021.
Frontline healthcare workers are among the first to receive the vaccine, and ophthalmologists and clinic staff could soon start receiving doses.

The effort is designed to empower patients, improve coordinated care, and reduce regulatory burdens.

The Association for Research in Vision and Ophthalmology announced the switch to a virtual format this week for its annual meeting, originally scheduled for May 2-6 in San Francisco.

The Centers for Medicare and Medicaid Services has released its final physician fee schedule for 2021. Despite opposition from the American Academy of Ophthalmology, the schedule includes an overall cut of 6% to ophthalmology.

In light of the ongoing COVID-19 pandemic, the Glaucoma Research Foundation will host a virtual Glaucoma 360 annual meeting in 2021.

During his 24-year tenure as chair of the Wayne State University School of Medicine’s department of ophthalmology and director of the Kresge Eye Institute, Dr. Jampel oversaw the growth of the department.
Thanks to researchers like Russell N. Van Gelder, MD, PhD, the possibility of restoring sight to patients blinded by age-related macular degeneration or retinitis pigments is another step closer to reality.

During AAO 2020, Cathy G. Cohen, MHSA, CAE, AAO vice president for governmental affairs, offered some optimism for the future of health care under the Biden administration.