
Approaches include anti-vascular endothelial growth factor agents, fine needle diathermy, and corneal collagen crosslinking.

Approaches include anti-vascular endothelial growth factor agents, fine needle diathermy, and corneal collagen crosslinking.
Performing cataract surgery on young patients can be a challenge, and the evolving technology can be used in cataract surgery for young children.

The company's AI-enabled mobile telemedicine platform helps clinicians capture retinal images and screen for vision-threatening eye diseases, such as diabetic retinopathy, glaucoma and age-related macular degeneration.

The company’s re:Vive technology features 6 vision diagnostic exams, supported by 5 reimbursable CPT codes in 1 wearable solution.
Investigators believe the therapy could prove to be an option for the treatment of other retinopathies.

Investigators set out to better define therapeutic options for controlling the inflammatory response and reducing the risk of pseudophakic cystoid macular edema after cataract surgery.

An experience at the grocery store serves as a reminder of the importance of fundraising for eye research.
Oxymetazoline hydrochloride has been recognized by the publication as a winner of its Best of Beauty Breakthrough Award.
Sotrovimab, a monoclonal antibody, appears to reduce the risk of progression of mild-to-moderate cases of COVID-19 in patients at high risk of progression.

The agreement also includes an exclusive option for Novartis to acquire Kedalion and its AcuStream technology. No financial terms were disclosed.
The Children’s Vision Equity Alliance was created to improve acces in children’s vision and eye health, working to advance equity in children’s vision and eye health through education, access, policies, and partnerships.

The 12-Month OMNI data from the prospective, multicenter GEMINI clinical trial show statistically significant postoperative reductions in mean IOP and suppression of daily IOP fluctuations.

A web app-based personalized music intervention before cataract surgery may lower anxiety levels or reduce the need for sedative medication.
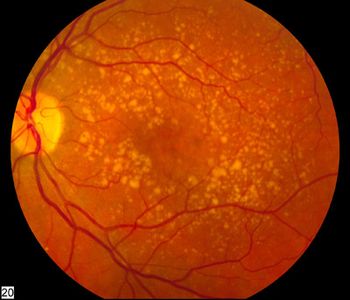
CellSight researchers at the Sue Anschutz-Rodgers Eye Center were published for a discovery that could lead to early diagnosis and intervention of dry age-related macular degeneration.

Veeral Sheth, MD, presents research finding faricimab, a bispecific monoclonal antibody, provided sustained retinal stability in patients with diabetic macular edema (DME) compared with other retinal treatments.
Shady Awwad, MD, discusses how the application of mitomycin C after corneal crosslinking does not prevent development of corneal haze after the procedure and actually contributes to development of more corneal haze.
Gareth Lema, MD, PhD, spotlights a treatment could provide benefits for patients with central retinal artery occlusion.

The results are in! Check out how your institution ranked in the 2021 Ophthalmology Times® Best Program Survey.

Bausch + Lomb's Yolande Barnard, shares an update on the FDA approval of XIPERE for the treatment of macular edema associated with uveitis.

Dilsher Dhoot, MD, reports results of the KESTREL and KITE trials, in which brolucizumab was compared with aflibercept for treating diabetic macular edema.

Investigators from the University of Maryland found that uncovering the mechanism of vision loss in Usher syndrome yields additional drug targets for eventual development of better therapies.
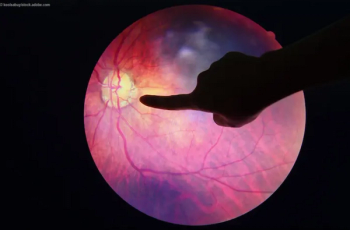
Unity Biotechnology announces improvement in visual acuity sustained through 24 weeks following single dose of UBX1325 in Phase 1 study of patients with advanced vascular eye disease.

Alcon this week announced its plans to acquire glaucoma surgery device maker Ivantis Inc. in a $475 million deal.

Perspectives from a pediatric ophthalmologist and mother.

Organizations are urging consumers to be aware of insurance policies that can limit their access to sight-saving procedures and treatments.
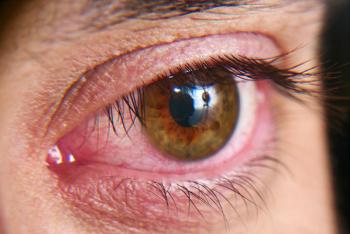
Aldeyra Therapeutics announced today that it has achieved the primary endpoint of ocular redness in its randomly assigned, double-masked, vehicle-controlled Phase 2 clinical trial in dry eye disease.

Allergan developed VUITY, which it noted is the first and only FDA-approved eye drop to treat presbyopia.
Investigators in Australia are developing a new rapid screening test for glaucoma that could help advance early detection of the disease.

The Cleveland Clinic named Rishi Singh, MD, president of the Cleveland Clinic Martin North and South hospitals in Martin County, Florida.

Two photographs are selected by an independent panel of five judges for this year’s honors.