
Investigators discuss the evolution of innovative technology for the hearing impaired and how vision can play a role.

Investigators discuss the evolution of innovative technology for the hearing impaired and how vision can play a role.
The doctors will join the Cataract and Primary Eye Care, Glaucoma, and Retina Services.

Several issues may restimulate the virus in those patients with prior exposure.
The Grail study finds the device can offer positive outcomes for patients.

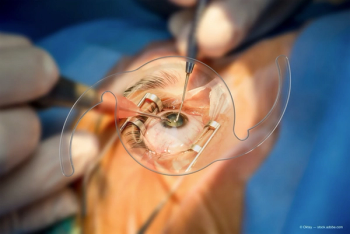
Bausch + Lomb has made an equity investment in Sanoculis and has entered into an exclusive European distribution agreement for MIMS minimally invasive surgical procedure.
Behin Barahimi, MD, discusses some current trends in the treatment of dry eye disease. An oculoplastic, orbital and reconstructive surgeon, she is a member of the faculty at Vanderbilt Eye Institute.
Oyster Point Pharma Inc. this week announced the expansion of patient access to varenicline solution (Tyrvaya) nasal spray and provided an update on the commercial performance of the spray in the United States. According to the company, its expanded patient access programs include more eligible patients with dry eye disease.

Gareth Lema, MD, PhD, discusses an innovative eye stroke program at Mount Sinai Health System that involves collaboration among ophthalmologists, the stroke service, and emergency department physicians.

Scientists at the Louisiana State University Health New Orleans Neuroscience Center of Excellence have developed a new, experimental human cell line from retinal pigment epithelial cells.
While the surgeons reported that the BW filter provided higher contrast during nucleus and viscous removal and posterior capsular polishing, they subjectively reported no significant advantage of using a BW filter.

Treatment and reduces burden of care, providing a treatment option for patients diagnosed with diabetic macular edema.

MyMD Pharmaceuticals Inc. announced recently that it has entered into a material transfer agreement with Bascom Palmer Eye Institute of Miami, Florida to collaborate on a pre-clinical study using MYMD-1 as a potential treatment for traumatic optic neuropathy.

According to the company, the study assesses the safety and tolerability of a single dose of CLS-AX administered to patients diagnosed with wet AMD by suprachoroidal injection.

Trial is evaluating the use of the Technolas TENEO excimer laser for vision correction surgery for hyperopia with astigmatism.
According to the company, new imaging options presenting opportunity for better outcomes for patients.
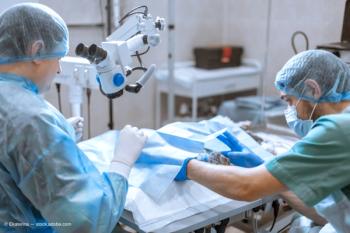
Results from the U.S. pivotal trial showed Apthera IOL subjects achieved statistically superior uncorrected intermediate and near vision, and equivalent distance vision and contrast sensitivity compared to control subjects.
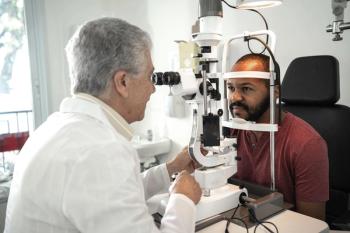
Mount Sinai study could lead to earlier and increased screening for this population to prevent blindness.

According to the company, 6-month safety and efficacy data are expected in Q1 2023. MCO-010 gene therapy reprograms healthy retinal cells to make them photosensitive.
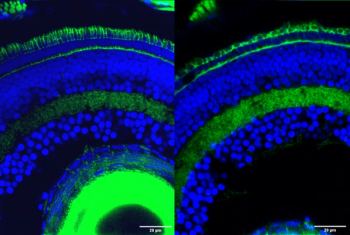
The findings could lead to a new understanding of unexplained causes of heritable retinal diseases.

Pediatric ophthalmologists may need background information when treating young patients.

LBS-008 is the company’s orally administered tablet for the treatment of Stargardt disease. There are currently no FDA approved treatments for Stargardt disease or dry AMD.
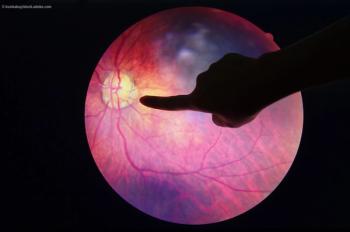
NIH-funded clinical trial finds that starting with a cheaper drug and switching to a more expensive drug as needed leads to good vision outcomes in diabetic macular edema.

A novel eye drop under development may provide neuroprotection to the retinal ganglion cells.

Physicians use a new administration method to treat patients diagnosed with noninfectious uveitis.
According to a team of investigators from Yale, Stanford and Dartmouth, the variances in regional healthcare spending are not limited to either public or private payers.
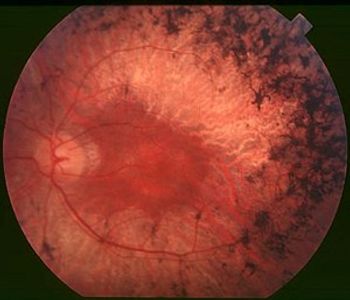
A team of scientists, led by Andrzej Foik, PhD, of the International Center for Translational Eye Research in Poland, is working on new therapies that may slow vision loss in patients diagnosed with retinal degeneration.

The companies will jointly develop 4D bio-fabricated corneal transplants for diseases that require endothelial keratoplasty and natural lenticule transplants.