Therapeutic Glaucoma
Latest News
Latest Videos
CME Content
More News

Therapeutic has fewer adverse effects in patients with POAG, OHT.

According to the company, its new 20,000-square-foot facility in Hayward, California, includes cGMP suites, developmental and testing laboratories, equipped with single use bioreactors.

Researchers at the Smurfit Institute of Genetics, in collaboration with the biotechnology company Exhaura Ltd., have shown that a gene therapy-based approach can decrease intraocular pressure in pre-clinical models of glaucoma.

Brimonidine Tartrate Ophthalmic Solution is an alpha-adrenergic receptor agonist indicated for the reduction of elevated intraocular pressure in patients with open-angle glaucoma.

SBI-100 Ophthalmic Emulsion is a synthetic cannabinoid derivative to treat glaucoma and ocular hypertension.

Thurein Htoo, MS, MBA, CEO and co-founder of Qlaris Bio, took the podium at the New Horizons Forum at the Glaucoma 360 conference in San Francisco to discuss the performance of the company’s episcleral venous pressure eyedrop, QLS-111.
Based on their findings, an international team of researchers is discouraging the application of atropine as a standard therapy for trabeculectomy surgery.

The company said it plans to generate clinical data aiming to demonstrate the potential retinal benefits of NCX 470 in order to add significant therapeutic value to the product profile in glaucoma.

Nanoscope Technologies LLC announced this week it has received Direct Phase II SBIR grant from National Institutes of Health for developing an innovative approach to autonomously regulate pressure for treatment of Glaucoma in a gene-agnostic manner.

Genotyping and artificial intelligence are together starting to predict the progression of glaucoma in individual patients, sparing them from suboptimal treatments and adverse effects. Recent research efforts are exploring modifiable risk factors such as caffeine consumption.
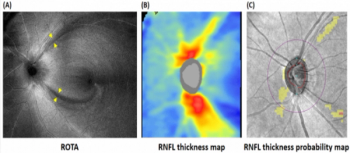
A research team led by the Department of Ophthalmology, School of Clinical Medicine, LKS Faculty of Medicine of The University of Hong Kong, with collaborators from the Faculty of Medicine of The Chinese University of Hong Kong and local and international partners, have developed a new technology ROTA to unveil the optical texture and trajectories of the axonal fiber bundles on the retina.

Timing of type 2 diabetes or hypertension diagnosis may impact the risk of primary open-angle glaucoma.

Bausch + Lomb has made an equity investment in Sanoculis and has entered into an exclusive European distribution agreement for MIMS minimally invasive surgical procedure.

Janet B. Serle, MD, discusses the pros and cons of various treatment options.

NCX 470 is currently enrolling patients in two multi-regional Phase 3 glaucoma clinical trials, Mont Blanc and Denali. The objective of these two trials is to demonstrate statistically superior IOP lowering of once daily dosed NCX 470
Device demonstrates potential as a durable, sustained-release glaucoma therapy
The company announced the expansion of its commercial rollout of its MIGS implant to the UK

The company is planning a Phase 2 trial with an optimized formulation in wet AMD that is expected to start in fourth quarter of 2022.
Vanderbilt’s David Calkins, PhD, was honored with the 2022 President’s Award, presented by the Glaucoma Research Foundation, at the Glaucoma 360 Annual Gala last month.

The glaucoma toolbox is expanding to include laser, drug delivery systems, and minimally invasive glaucoma surgery.
Mark Gallardo, MD, reports 12-month outcomes of iStent inject® combined with cataract surgery in a predominantly Hispanic patient population with mild to moderate open-angle glaucoma (OAG) and comorbid cataract.
Basil Williams, Jr., MD, reports on the association between development of elevated intraocular pressure and numerous commonly performed vitreoretinal treatments.
Although minimally invasive procedure gives hope, additional research is required.
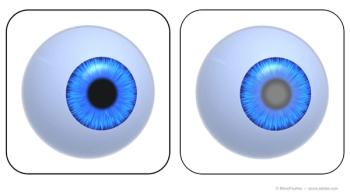
Pressure-dependent optic nerve disease, retinal disease.































