
Variable TULP1 missense mutations in inherited retinal diseases.


Variable TULP1 missense mutations in inherited retinal diseases.

Greg Kunst, president and CEO of Aurion BioTech, shares a company update on endothelial cell therapy trials, clinical plans and upcoming data.

John Berdahl, MD, discusses the technique and benefit of endothelial cell therapy. An early technology, this therapy method allows for up to 100 treatments out of a single human cornea.

Impact of native lipids on rhodopsin signaling and regeneration opens door to GPCR drug discovery in native membrane environments

The trial marks the first-ever in vivo delivery of an experimental CRISPR gene editing medicine to a pediatric patient, with the company on track to complete dosing of the pediatric mid-dose cohort in the first half of 2022.

According to researchers at the University of California, Irvine, base editing may provide long-lasting retinal protection and prevent vision deterioration in patients with inherited retinal degeneration, specifically in Leber congenital amaurosis patients.

According to the company, the modifier gene therapy candidate is for the treatment of retinitis pigmentosa resulting from mutations in the nuclear receptor subfamily 2 group E member 3 and Rhodopsin genes.

The company’s announcement marks first clinical trial in humans of Ocugen’s modifier gene therapy platform.

A team of investigators at the Okinawa Institute of Science and Technology Graduate University in Japan have identified a gene necessary for the survival of retinal ganglion cells – a class of neurons located in the retina that are critical for vision.

Investigators find that hybrid cells could be a potential therapeutic strategy to treat retinal damage and visual impairment.

A team of investigators has found that nomacopan, a recombinant biologic derived from blood-feeding ticks, may be an option for the treatment of allergic eye disease due to its ability to down-regulate the LTB4/C5 pathways in experimental allergy conjunctivitis.
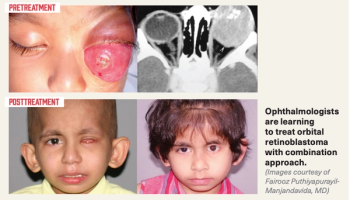
Investigators observe survival rate with orbital retinoblastoma improves substantially due to a combination approach that includes intensive sequential treatment comprised of chemotherapy, enucleation, and external beam radiation therapy.

According to the company, pegcetacoplan demonstrated continuous and clinically meaningful effects at month 18 in the studies, which also found that treatment effects in DERBY were comparable to OAKS during months 6 to 18. The combined 18-month data show the potential for improving treatment effects over.
A gift from Bronwyn Bateman, MD, will establish the UCLA Bronwyn Bateman Center for Ocular Genetics at the Stein Eye Institute.
A team of investigators has found that the eye disease is more prevalent than first thought.

Trial recruitment is enabled by the Foundation Fighting Blindness' patient registry, My Retina Tracker Registry, which includes gene

The clinical trial will evaluate the safety and tolerability of ADX-2191 in patients diagnosed with RP due to mutations of the rhodopsin gene, including the P23H gene mutation.

Christina Y. Weng, MD, MBA, an associate professor of ophthalmology and surgical retina fellowship program director at Baylor College of Medicine in Houston, recently shared some standout therapies for macular degeneration.

The companies note that the partnership will propel Ray Therapeutics’ lead optogenetics gene therapy to Phase 1-2 clinical trials.

Investigators at Trinity College Dublin have highlighted the SARM1 gene, a key driver in the damage that ultimately leads to impaired vision.

In this episode of EyePod, Ora Clinical’s Keith Lane offers a brief overview of the current state of ophthalmology and its standardized endpoints. He also discusses some of the challenges that gene therapy has faced, particularly in relation to ophthalmology. He also offers his outlook for the future of gene therapy.

Dr. Carel Hoyng divulges the main takeaways from his Angiogenesis presentation, including the origins of Stargardt disease, correct diagnosis, ongoing gene therapy trials and the future of therapy.

Carel B. Hoyng, MD, noted that investigators have developed an RNA therapy to stop the progression of the disease, which ultimately leads to legal blindness.
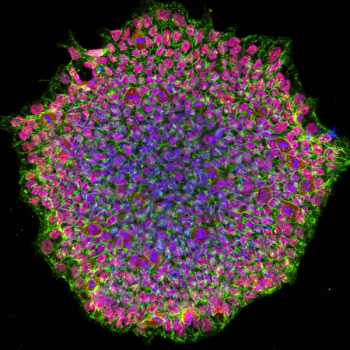
The team hopes the use of patient-derived stem cells will enable high-throughput drug screening for potential therapeutics for patients.

Cleveland Eye Bank Foundation (CEBF) will feature nine researchers from three eye institutions on February 15, from 3 to 5 pm Eastern, for its second annual virtual vision research symposium.