AMD
Latest News

Latest Videos

CME Content
More News
In a study, a team of Korean researchers developed an AI model using OCT images to predict neovascular AMD treatment outcomes after anti-VEGF injections. The model highlights AI’s potential in personalized ophthalmic care.
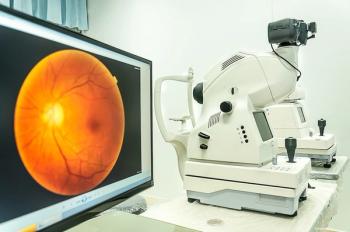
A study suggests that small hyperreflective retinal foci (HRF) on OCT images, linked to activated microglial cells, could serve as a valuable marker for monitoring neuroretinal inflammation and progression in age-related macular degeneration.

ABI-110 has the potential to offer a durable and effective solution by addressing the root causes of wet AMD at the genetic level.

The CHMP has recommended FYB203 for approval in Europe for treating adult patients with age-related neovascular macular degeneration (nAMD) and other serious retinal diseases.

After closing, the combined company is expected to operate under the name Kalaris Therapeutics, Inc.

Both LUNA and OPTIC were designed to assess a broad wet AMD population, including hard-to-treat patients with severe disease who required frequent anti-VEGF injections before enrolling in the trial.

The California Institute for Regenerative Medicine has funded Keck School of Medicine of USC translational research advancing a therapy for dry age-related macular degeneration, one of the leading causes of blindness in older adults.

Afqlir (aflibercept) is a 2 mg vial kit and pre-filled syringe for intravitreal injection.

The drug is the world’s first-ever CRISPR/Cas13 RNA-editing therapy for clinical use in treating neovascular age-related macular degeneration (nAMD).
A Johns Hopkins study reveals that anti-VEGF treatments for wet AMD may unintentionally elevate ANGPTL4, a protein that promotes blood vessel growth. Combining anti-VEGF with an experimental drug targeting HIF-1 could enhance vision outcomes and prevent vision loss.

Scientists at Scripps Research and its nonprofit drug development arm, the Calibr-Skaggs Institute for Innovative Medicines, are working to advance regenerative medicines capable of repairing tissues damaged by age-related disease

The Valeda Light Delivery System is for the treatment of patients with dry age-related macular degeneration (AMD).

In an interview with David Hutton of Ophthalmology Times, Ash Abbey, MD, discusses the Phase 3 LUGANO trial for EYP-1901 in treating wet age-related macular degeneration. Abbey discusses the trial’s non-inferiority objectives, treatment schedule, patient outcomes like reduced treatment burden, and anatomical stability. Patient interest is high, particularly among those wanting fewer injections, though recruiting treatment-naive patients may be challenging. If results are favorable, potential FDA approval could occur within 3 to 4 years.

Researchers receive $6.4 million grant from NEI to seek new treatment for blindness-causing diseases
Researchers receive $6.4 million grant from NEI to seek new treatment for blindness-causing diseases.

CBNS, such as turmeric, are natural anti-inflammatory and antioxidant agents that may confer benefits against AMD.

According to the company, the second Phase 3 LUCIA pivotal trial first patient dosing is expected by end of 2024 with topline data anticipated in 2026.

According to a study presented at the American Academy of Ophthalmology’s annual meeting in Chicago, investigational treatment reduced vision loss and protected key structures in the eye essential for vision.
The subretinal drusenoid deposits may serve as a biomarker of serious heart disease.

According to the company, the axitinib injectable suspension achieved all primary and secondary outcomes and maintained stable visual acuity and anatomical control over 9 months.
Related macular dystrophies include Sorsby’s fundus dystrophy, Doyne honeycomb macular dystrophy, and autosomal dominant radial drusen.

The company made notable hires in its biometrics, clinical operations, and market access departments.
The data were presented at the Keystone Symposium Targeting Dry Age-related Macular Degeneration: Pathophysiology and Emerging Therapies.
Socioeconomic factors have long been a looming issue in various health outcomes. A team of researchers from Shanghai Jiao Tong University School of Medicine and Fudan University set out to review the impact of socioeconomic status inequality on the incidence of AMD.

Oral gildeuretinol demonstrated a clinically meaningful reduction in the geographic atrophy lesion growth rate at 24 months, supporting additional clinical development. SAGA topline data has been accepted as a late breaker at the 128th annual meeting of the American Academy of Ophthalmology, being held October 18-21 in Chicago.