Cornea
Latest News
Latest Videos

CME Content
More News

Results of the study indicated that systemic treatment led to elevations in liver function test results, with some patients experiencing values twice as high as normal.

Researchers conducted a retrospective chart review spanning from January 2015 to January 2021 to compare the incidence rates of infectious keratitis before and during the pandemic among PRK patients.
Ula Jurkunas, MD, sat down to discuss her presentation on the management of anterior corneal disease at this year's ASCRS meeting held in Boston, Massachusetts.

The company announced it will share clinical data at Eyecelerator and the American Society of Cataract and Refractive Surgery 2024 Annual Meeting in Boston.
The 1-time, intracameral injection is a cell therapy for the treatment of corneal edema secondary to corneal endothelial dysfunction.

Alice Epitropoulos, MD, discusses the importance of considering ocular surface health in cataract surgery patients.

Alexander Movshovich, MD, PhD, a specialist in keratopigmentation, sat down to talk about the controversy surrounding the procedure after it went viral on social media.

Early keratoconus screening advocated
Shifting the treatment paradigm can lead to less pain and inflammation.

The funding has been awarded to BIENCO, which is working to cure one of the most common causes of blindness in the world by developing cost effective, individually tailored, superior corneas.

OK-101 is the first IND clearance granted by the FDA for a drug to begin clinical studies specifically to treat patients suffering with neuropathic corneal pain.

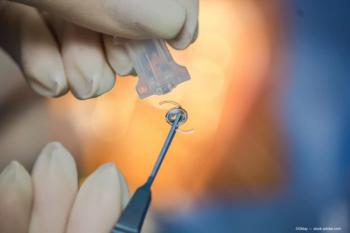
Patient selection is important.

Researchers found the administration of the neuropeptide α–melanocyte–stimulating hormone promotes corneal healing and restores normal eye function to an otherwise degenerating and diseased cornea by providing protection against cell death and promoting cell regeneration.

Physicians from the Harvard Medical School and the Kresge Eye Institute at Wayne State University in Detroit recently published details from the case.

Approach to lowering IOP reduces medication burden without risking graft rejection.

Sterile corneal ulceration is a rare complication of rheumatoid arthritis and may lead to corneal perforation. However, surgeries performed to restore vision often are not successful.
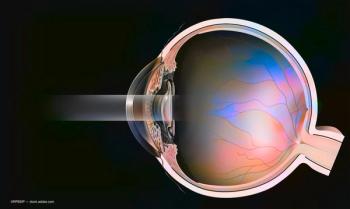
Physician notes endothelial cells play key role in hydration, transparency of cornea.
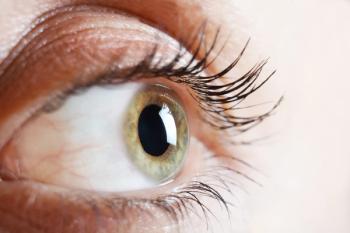
Acanthamoeba keratitis can lead to inflammation of the cornea and result in extreme levels of pain, light sensitivity and vision loss.

The ANTERION from Heidelberg Engineering offers a range of advanced diagnostic tools, including IOL power calculation, corneal topography, and tomography.

Held the second Thursday each October, World Sight Day is an annual day of awareness to focus global attention on blindness and vision impairment.

The team focused on the research pf the senescent phenotypes of human corneal and conjunctival epithelial cells.

According to the company, study enrollment is planned to commence during Q1 2024 following IND allowance by the FDA.
Steven A. Greenstein, MD, emphasizes the importance of identifying and assessing eye emergencies, highlighting the need to determine when to escalate care from primary to subspecialty settings. He discusses various types of anterior segment emergencies, while stressing the significance of maintaining a calm demeanor in these critical situations.