
Colman R. Kraff, MD, shares highlights of a study evaluating the safety and efficacy of the use of the iDesign 2.0 Refractive Studio for the treatment of patients with hyperopia and myopia.

Colman R. Kraff, MD, shares highlights of a study evaluating the safety and efficacy of the use of the iDesign 2.0 Refractive Studio for the treatment of patients with hyperopia and myopia.
Chuck Hess shares an update on Bausch + Lomb's latest developments in surgical technology, as well as how the company has adapted its operations during the pandemic.

Jag Dosanjh, senior vice president of U.S. Eye Care, Allergan, an AbbVie company, offers a preview of the company's latest innovations, developments, and what attendees can look forward to at ASCRS 2021.
AAO, Surgical Care Coalition teaming up to ensure the final version of the rule is fair to ophthalmologists and surgeons as one company is expressing its disappointment in the proposal.

Nanoscope Therapeutics Inc. has dosed its first patient in a late-stage Phase 2b trial of a gene therapy that delivers multi-characteristic opsin to retinal cells.
The Gilbert Family Foundation is collaborating with the Children’s Oncology Group and Children’s Hospital of Philadelphia to validate a tool to measure progressive vision loss.
According to iSTAR Medical, the STAR-V study will investigate the device in 350 patients with primary open angle glaucoma.
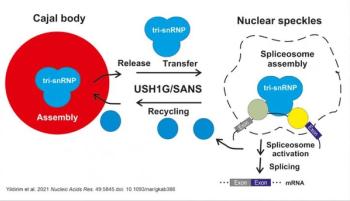
Usher syndrome 1G protein SANS regulates the splicing of genes, particularly those linked to Usher syndrome, which can lead to early vision loss form RP.

According to Unity Biotechnology, its trial for the small molecule inhibitor of Bcl-xL found improvement in visual acuity and central subfield thickness observed in diabetic macular edema and wet age-related macular degeneration patients treated with UBX1325.

According to investigators, the system has been shown to almost triple the number of people with eye problems attending primary care, as well as increasing appropriate uptake of hospital services.
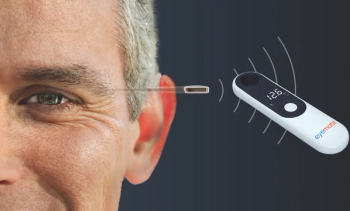
Implandata received FDA Breakthrough Device Designation for its Eyemate-SC in April and now has CE Mark approval in the European Union.

According to investigators, the new technology is designed to detect telltale signs of major blinding diseases in retinal blood and tissue that typically go unseen until it is too late.

The US Supreme Court ruled Thursday that the Affordable Care Act remains valid, rejecting a claim by several states that a recent change to the law made it unconstitutional.

The event, being held at the Mandalay Bay Resort and Casino in Las Vegas, is offering a full agenda for in-person attendees.
Classification criteria funded by the National Institutes of Health will facilitate clinical research for new therapies.

Stuart will enroll 150 volunteers in the study, which is being conducted with the support of Stuart's strategic partner and CRO, Ora Clinical. ST-100 is based on PolyCol, the company's synthesized polypeptide tissue reparative platform.

Physicians want to make sure they see each their patient frequently enough not to miss important changes in eye disease.

According to preliminary results, intranasally delivered ST266 is a safe treatment option.

Nanoscope Therapeutics Inc. expects to advance the therapy by launching a late-stage Phase 2b trial this summer with gene therapy that delivers multi-characteristic opsin to retinal cells.

Upsher-Smith Laboratories LLC and Rafarm SA have announced the U.S. commercial launch of moxifloxacin ophthalmic solution, USP 0.5% following a recent abbreviated new drug application (ANDA) approval by the FDA.

The company is hoping to commercialize the first FDA-approved ophthalmic formulation of bevacizumab-vikg for retinal disease. It expects to receive BLA approval from the FDA by mid-2022.
A new report by George Washington University offers a snapshot of health care workers during the COVID-19 pandemic and offers recommendations to prepare for the future.
Backed by a five-year, $6.4 million grant from the National Eye Institute of the National Institutes of Health, investigators from Case Western Reserve University, University Hospitals and the Jaeb Center for Health Research hope to determine which diabetic patients can successfully donate their corneas for keratoplasty (and which should not).
The company plans to start a second Phase 3 registration study, VISION-2, as it continues on path toward a New Drug Application submission to the FDA.

A team of researchers at the University of Cincinnati's Department of Chemistry and Department of Ophthalmology, in collaboration with researchers at Cincinnati Children's Hospital Medical Center and the Ohio State University, have received a four-year, $1.6 million grant from the National Institutes of Health/National Eye Institute to develop a drug delivery system that is more efficient and longer lasting than conventional eye injections.

An international research team has shown that optogenetic therapy has helped to partially regain visual function in a patient with retinitis pigmentosa. This is a milestone towards a gene therapy that could restore vision.

There are more people with vision loss and blindness than previously estimated, according to a new study supported by Prevent Blindness.

After a US District judge rules against request for preliminary injunction, Johnson & Johnson Vision moves forward with litigation in several countries, including the United States, to resolve intellectual property disputes against Alcon.

While Biogen’s XIRIUS study did not meet its primary endpoint of demonstrating a statistically significant improvement in treated eyes, positive trends were observed across several clinically relevant prespecified secondary endpoints.
A study led by investigators at St. Michael's Hospital of Unity Health Toronto supports pneumatic retinopexy as a primary retinal reattachment technique to achieve better long-term integrity of photoreceptors.