
A team of researchers from Thomas Jefferson University have found that immune cells could be doing much more than first thought in protecting our eyes.

A team of researchers from Thomas Jefferson University have found that immune cells could be doing much more than first thought in protecting our eyes.

The cell-by-cell atlas will help in the study of eye disorders and development of cell therapy to replace damaged eye tissue.

According to the company, a suprachoroidal injection of CLS-AX 0.1 mg dose was well-tolerated in cohort 2 with no treatment related adverse events, while the consistent safety profile observed in in cohorts 1 and 2 supports advancement to cohort 3.

Researchers found there was, on average, a 17% improvement in participants’ color contrast vision when exposed to three minutes of deep red light in the morning.
Ahmad Aref, MD, and Paul Singh, MD, offer a recap of their EyeCon 2021 presentation.
The company continues to develop testing options that deliver accurate detection and correctly identify SARS-CoV-2 mutations to help patients and healthcare providers manage the evolving COVID-19 pandemic.

The company anticipates that the Sirius and Celeste studies will further validate QR-421a’s ability to stabilize vision loss in people with USH2A mediated retinitis pigmentosa and Usher syndrome.
The US Orphan Drug Designation would provide seven-year market exclusivity if Visomitin was approved for use in LHON.

Researchers at the University of Virginia School of Medicine have made a discovery linking lupus, a potentially debilitating autoimmune disorder, and macular degeneration, a leading cause of blindness.

According to the company, OK-101 displays both anti-inflammatory and ocular pain-reducing potential.

I-MED Pharma Inc., a Canadian-based firm, announced this week the expansion of its strategic partnership with ESW Vision in France to be their exclusive distributor in the United States.

TearLab Corp. announced that Adam Szaronos has been appointed president and CEO by the company's Board of Directors, effective immediately.
Novartis has revealed the first interpretable results from year two (week 100) of the Phase III KESTREL study.
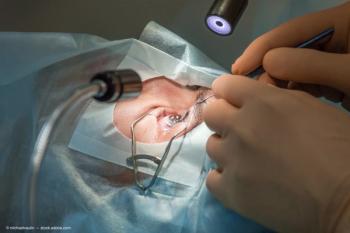
Under terms of the agreement, ImprimisRx to assume full responsibility for US sales and marketing activities for Dexycu.

According to the company, the board will support the advancement of late-stage ophthalmic assets Nyxol and APX3330.

Gift marks the first time the ACMG Foundation has allocated an award for training in ophthalmic genetics.

The company’s IOPerfect combines artificial intelligence in a VR-like headset to allow tele-diagnosis and remote monitoring of glaucoma.
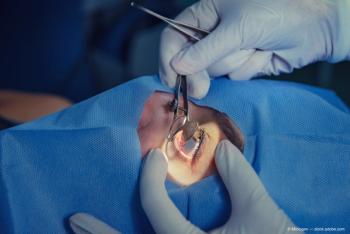
The company will begin commercialization of the IC-8 small aperture IOL, used for cataract patients, upon successful completion of the manufacturing facility inspections and receipt of an official approval order from the FDA, which the company estimates in Q2 2022.

The agreement includes Europe, Commonwealth of Independent States countries, China, India, parts of Latin America and the Oceania countries.

Researchers have now found that subjects who underwent cataract surgery had nearly 30% lower risk of developing dementia from any cause compared with those who did not.

Study shows statistically significant improvement for primary endpoint of bulbar conjunctival hyperemia for OTX-DED 0.2 mg and 0.3 mg formulations compared with vehicle hydrogel insert.
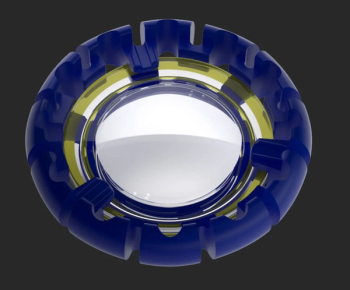
The approval will allow LensGen to move forward with the IDE pivotal trial with the ultimate goal of seeking premarket approval in the United States.

More than 200 medical associations, including the American Medical Association and the Medical Group Management Association, have co-signed a letter seeking Congressional action on the issue before the end of the year.

AMA President Gerald E. Harmon, MD, said in a statement that President Joe Biden’s pandemic plan will make communities safer amid an evolving pandemic and confirmed cases of the Omicron variant.

Omidria will become a key product in Rayner's ophthalmology franchise, which includes intraocular lenses, ophthalmic viscoelastic devices and dry eye treatments.
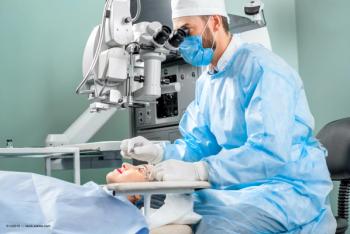
The novel sustained-release technology has potential to eliminate the need for eye drops after cataract surgery.

Using its proprietary software, the company has been able to create models in a fraction of the time typically needed for fitting one.

The company's AI-enabled mobile telemedicine platform helps clinicians capture retinal images and screen for vision-threatening eye diseases, such as diabetic retinopathy, glaucoma and age-related macular degeneration.

The VIVID study focuses on three novel topical ophthalmic formulations under investigation for the treatment of presbyopia

The company’s re:Vive technology features 6 vision diagnostic exams, supported by 5 reimbursable CPT codes in 1 wearable solution.