
A group of French researchers is hoping its work to develop an artificial retina will help blind people see again.

A group of French researchers is hoping its work to develop an artificial retina will help blind people see again.

Phase 2 trial of 0.3% minocycline microparticle suspension shows promise in dry eye diseases associated with Inflamed meibomian gland dysfunction

RegeneRx Biopharmaceuticals announces that its ARISE-3 Phase 3 clinical trial evaluating RGN-259 eyedrops did not meet its primary outcome measures.

According to a national study, serious vision issues among older Americans have dropped sharply, with the greatest improvement found among women, individuals over 85 and Black or Hispanic seniors.
Researchers at the Royal College of Ophthalmologists offer guidance on the impact of treatment delays in crosslinking (CXL) amid COVID-19 on patients with keratoconus.
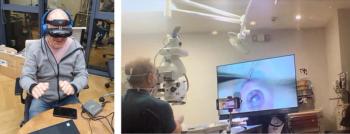
A Canadian artificial cornea clinical trial has been enhanced by a remote, real-time 3D HD video training solution.
The study will evaluate the safety and tolerability of the R:GEN laser in patients with early-stage age-related macular degeneration.
Citing an unstable supply chain, Pfizer is discontinuing the ophthalic drops.

George Marcellino, vice president of Iridex, is being remembered by colleagues as a man of substance.

Topcon Corp. will purchase exclusive rights to distribute Iridex Corp.’s retina and glaucoma products, while also acquiring a common equity stake in the company. Iridex will acquire Topcon’s PASCAL product line.

With funding from the National Eye Institute, the team of researchers will focus on developing a broad-spectrum immunomodulatory eye drop for patients dealing with dry eye and ocular surface disease stemming from inflammatory and immune system disorders.

The bulk of a $25 million gift from the Henry L. Hillman Foundation will drive vision care research and development through The Eye and Ear Foundation and the University of Pittsburgh Departments of Ophthalmology and Otolaryngology.
According to a survey by the Larry A. Green Center, in collaboration with the Primary Care Collaborative (PCC) and 3rd Conversation, primary care offers assets, including experience and relationships with patients.

Gene therapies to mitigate the effects of mutations that cause blindness are undergoing rapid development. Investigators have found that novel gene vectors reduce the risks associated with these procedures.

Dr. Bath could be the first African-American woman inducted into the Hall. As the first African-American woman to receive a medical patent, for the laserphaco probe, and the first woman to lead a post-graduate training program in ophthalmology, her experience is extensive.
The drop, formerly prescribed as Pazeo, joins Alcon’s over-the-counter ocular allergy portfolio to meet a variety of patient needs.

Applicants must submit an original essay focusing on how biologics are transforming regenerative medicine in eye care. The deadline to apply is March 12.
New World Medical relied on feedback from surgeons in the development of the device, which was registered with the FDA in October.

LumiThera has announced positive interim data in visual and ERG clinical outcomes in the ELECTROLIGHT pilot study in dry AMD patients.

Investigators are studying the use of photobiomodulation in the treatment of dry age-related macular degeneration.

Following a successful Phase 2 study, a Phase 2/3 clinical trial for the compound is currently planned for mid-2021.

Investigators from the University of Pittsburgh and West Virginia University will collaborate on an international glaucoma laser study funded with a combined $15.2 million grant.

Paul S. Bernstein, MD, PhD, leads a team of University of Utah investigators that has developed a method for synthesizing large enough quantities of very-long-chain polyunsaturated fatty acids to evaluate their potential sight-preserving properties.

The American Society of Cataract and Refractive Surgery has changed the venue for its 2021 annual meeting. The conference will now be held July 23-27 at the Mandalay Bay Convention Center in Las Vegas.
The study focused the safety and tolerability of increasing doses of polihexanide eye drops in order to choose a dose that would be tested in a phase III clinical trial in patients with acanthamoeba keratitis.
Glaukos Corporation's COO Chris Calcaterra gives an update on the company's latest 24-month phase 2b data for its iDose TR sustained-release travoprost implant, next steps for securing FDA approval, and his outlook for 2021.

Donald L. Budenz, MD, MPH, shares the key takeaways from his Shaffer-Hetherington-Hoskins Lecture, focusing on glaucoma epidemiology, presented during the 2021 Glaucoma 360's 25th Annual Glaucoma Symposium CME.

The Phase 1 clinical trial of EyePoint Pharmaceuticals Inc.’s EYP-1901 is underway. It is a potential twice-yearly sustained delivery anti-VEGF treatment targeting wet age-related macular degeneration.

Robert L. Stamper, MD, discusses the findings of a study analyzing the effects of corneal mechanics on IOP, presented during the virtual Glaucoma 360 meeting.

During the 2021 Glaucoma 360 meeting, Ruth D. Williams, MD, speaks on the highlights and key findings from the EAGLE study, which showed clear lens extraction surgery may be a better initial treatment for some glaucoma patients.