
When exploring novel treatment options for the management of IOP, clinicians should consider a number of independent factors ranging from beneficial behaviors to body position.

When exploring novel treatment options for the management of IOP, clinicians should consider a number of independent factors ranging from beneficial behaviors to body position.

When it comes to recommending a premium IOL, a patient’s glaucoma is only one factor to consider. Just like any other patient planning cataract surgery, visual needs and preferences for/against glasses are also important factors.
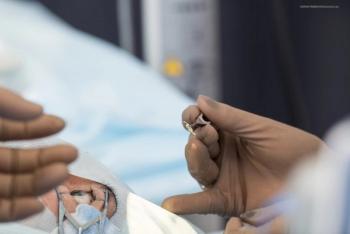
Residual astigmatism is not uncommon after toric IOL implantation. Depending on its cause and magnitude, lens reorientation may be a good solution.

A 4-year study following trifocal IOL implantation (AT LISA tri 839MP, Carl Zeiss Meditec) found that the lens provided good distance, near, and intermediate visual acuity. The lens also improved patient quality of vision with better diffraction and less reduction in contrast sensitivity.

One thing has remained constant since the inception of Google: the importance of unique and compelling content. So, the answer seems to be just keep adding content to your website-right? Unfortunately, no.

Cataract and refractive surgeons should use prophylaxis to avoid reactivation of the herpes simplex virus (HSV) in patients with a history of infection with this virus, according to Elizabeth Yeu, MD.

Tomasso, a vitreoretinal surgeon, recently shared a blog by someone who calls him/herself “Neuroskeptic”.Neuroskeptic penned a spoof “scientific” article about midichlorians, which are the little organisms inside cells that give Jedi Knights (the good guys in the “Star Wars” movies) their powers (and unfortunately, confer those same powers to certain bad guys, like Darth Vader).

Having explored the various options available for micro-invasive glaucoma surgery (MIGS), I now use several devices with success. One MIGS device (XEN Gel Stent, Allergan) differs from the others in that it drains aqueous into the subconjunctival space and can be performed as a stand-alone procedure without cataract surgery or combined with cataract surgery.

Assessing visual field progression in glaucoma may be more of an art than a science-and there is no one test to give reliable information that is needed about a patient, said Chris A. Johnson, PhD, DSc, FAAO, FARVO, professor, Department of Ophthalmology, and director, Visual Field Reading Center, University of Iowa, Iowa City.

Juan Carlos Izquierdo, MD, decribes how CO2 laser-assisted sclerectomy surgery is a successful, less invasive, and more simple option for a wide range of glaucoma patients. Due to his overall success with the CLASS procedure, he no longer performs trabeculectomy.

Advancing methods for evaluating therapeutic response have fostered a new era in glaucoma neuroprotection in which promising candidates are already being investigated in clinical trials and others are poised to begin phase I testing, said Jeffrey L. Goldberg, MD, PhD.

When setting a target IOP for patients with glaucoma, consideration of IOP fluctuations and the degree to which they vary is important.

With its ability to restore the eye’s natural outflow pathway, ab-interno canaloplasty (ABiC) presents a viable option for the treatment of different stages and severities of glaucoma, according to Mark J. Gallardo, MD.

It is well known that with most disease, the earlier it is detected, the better the outcome prognosis. Along with early detection comes the need for early treatment. Traditionally, options for glaucoma have been limited. Medications can be effective when used properly, though patients are notorious for compliance issues.

A dysfunctional clinic environment may mean the power lies with employees instead of the administration. Be aware of common complaints so the power of the practice can be taken back.

Published in the American Journal of Ophthalmology, a retrospective case-controlled study between cohorts of rapid glaucoma disease progressors and nonrapid progressors found that rapid progressors were older, had significantly lower central corneal thickness, and baseline intraocular pressures.
In the past year, Matossian Eye Associates added a new category of presbyopia-correcting IOLs to the practice: the extended depth of focus (EDOF) IOL. The first IOL in this category is the Tecnis Symfony (Johnson & Johnson Vision). EDOF lenses from other manufacturers are in clinical trials, so it behooves the cataract surgeon to better understand how these lenses work.

Ophthalmologists all know how to safely watch an eclipse, but how will ophthalmologists around the country be participating in and observing the event?

Antibiotic resistance against fluoroquinolones is increasing, and that may adversely impact patients with retinal disorders. The introduction of intravitreal injections (IVT) to treat retinal diseases has increased from about 1 million in 2007 to an expected 6 million in 2016 (up from only 4,000 in 2001).

Ophthalmology Times asked readers for insights on antibiotics and eye infections from clinicians in the field - including experience with postoperative infections after eye surgery, if you use topical antibiotic prior to cataract surgery, how you choose antibiotics in a routine surgical prophylaxis, and more. The 118 U.S.-based ophthalmologists who responded were entered into a drawing to win a $200 gift card. Here are the survey results.

It will soon be time for the FIL – Feira Internacional de Lisboa in Lisbon, Portugal to open its doors to the ophthalmic community for the 35th Congress of the European Society of Cataract and Refractive Surgeons (ESCRS), taking place between the 7th and 11th October 2017.

Faced with an increasingly competitive environment, eye surgeons need to appeal to younger patients. The way to accomplish this is relatively straightforward: Be where they are. Of course, the more challenging part of this equation is “How?”

Oral cetirizine is one of the most used oral medications for treatment of allergic rhinitis. In May 2017, the FDA approved the first ophthalmic formulation of the second-generation histamine-1 (H1) receptor antagonist for use in treating ocular itching associated with allergic conjunctivitis.

Findings in the journal Clinical Ophthalmology report that using the technique phacoemulsification was performed without complications in 607 of 609 cataract eyes.

My friend, Dave, an oculoplastic surgeon, trained the same time I did. He was extremely intelligent and possessed a great sense of humor. A real jokester, he provided free cosmetic services to his office staff, and claimed in their presence that his treatments prevented them from frowning at him.

Implantation of an investigational device for presbyopia resulted in a minimal 2-line increase in distance-corrected near visual acuity and an actual reduction in the amount of near add needed over time, show findings from a single-center, subgroup analysis.

A comparative analysis suggests placement of a cornea inlay (Kamra, AcuFocus) has improved patient satisfaction, refractive stability, and visual results when placed at 250 μm or deeper in the cornea. Shallower implantation depths may be more prone to refractive instability and lower patient satisfaction.

The use of epi-on photorefractive intrastromal cross linking reduced myopia by about 0.25 D in a small study; In a high-oxygen group, the effect averaged 1.25 D.

Hyperopic SMILE is being investigated in a prospective study. Early outcomes show good efficacy, safety, and predictability along with some interesting differences compared with hyperopic LASIK.

Wavefront-guided LASIK and wavefront-guided PRK following previous keratorefractive surgery demonstrate similar safety, efficacy, and predictability and result in comparable wavefront outcomes.