Researchers seek to identify new serological biomarkers that could better characterize Sjogren’s syndrome and overcome the limitations of traditional antibodies.

Researchers seek to identify new serological biomarkers that could better characterize Sjogren’s syndrome and overcome the limitations of traditional antibodies.

According to the company, preclinical study results from a 42-day proof-of-concept in vivo study demonstrated a single, intralacrimal gland injection of an adeno-associated virus containing the NGF gene produced statistically significant increase in NGF in tear film, as compared to placebo, as early as Day 7.

More Americans have used and continue to use teleheath for mental health care. according to a recent online poll by the American Psychiatric Association.

Upsher-Smith Laboratories LLC and Rafarm SA have announced the U.S. commercial launch of moxifloxacin ophthalmic solution, USP 0.5% following a recent abbreviated new drug application (ANDA) approval by the FDA.

The company is hoping to commercialize the first FDA-approved ophthalmic formulation of bevacizumab-vikg for retinal disease. It expects to receive BLA approval from the FDA by mid-2022.
A new report by George Washington University offers a snapshot of health care workers during the COVID-19 pandemic and offers recommendations to prepare for the future.
Vyluma Inc. will focus on the development and commercialization of therapies to treat ophthalmic diseases, including pediatric myopia.
Backed by a five-year, $6.4 million grant from the National Eye Institute of the National Institutes of Health, investigators from Case Western Reserve University, University Hospitals and the Jaeb Center for Health Research hope to determine which diabetic patients can successfully donate their corneas for keratoplasty (and which should not).
The company plans to start a second Phase 3 registration study, VISION-2, as it continues on path toward a New Drug Application submission to the FDA.

A team of researchers at the University of Cincinnati's Department of Chemistry and Department of Ophthalmology, in collaboration with researchers at Cincinnati Children's Hospital Medical Center and the Ohio State University, have received a four-year, $1.6 million grant from the National Institutes of Health/National Eye Institute to develop a drug delivery system that is more efficient and longer lasting than conventional eye injections.

An international research team has shown that optogenetic therapy has helped to partially regain visual function in a patient with retinitis pigmentosa. This is a milestone towards a gene therapy that could restore vision.
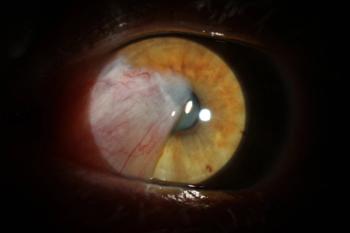
A recent study may indicate the need for future research to assess the relationship between pain and the anterior chamber depth.

Dexamethasone implant is key to treating non-infectious posterior segment uveitis.

Investigators at Mount Sinai report that ocular cells may be infected directly by the virus, with the limbus especially susceptible.

There are more people with vision loss and blindness than previously estimated, according to a new study supported by Prevent Blindness.

The U.S. Patent and Trademark Office issued today two patents to Ocuphire Pharma covering the late-stage product candidate Nyxol (phentolamine mesylate).

After a US District judge rules against request for preliminary injunction, Johnson & Johnson Vision moves forward with litigation in several countries, including the United States, to resolve intellectual property disputes against Alcon.

While Biogen’s XIRIUS study did not meet its primary endpoint of demonstrating a statistically significant improvement in treated eyes, positive trends were observed across several clinically relevant prespecified secondary endpoints.

According to investigators, understanding the ocular manifestations in patients with COVID-19 could ultimately help with the diagnosis and prevent transmission.
A study led by investigators at St. Michael's Hospital of Unity Health Toronto supports pneumatic retinopexy as a primary retinal reattachment technique to achieve better long-term integrity of photoreceptors.

Ocuphire Pharma has completed enrollment in the VEGA-1 Phase 2 clinical trial evaluating the safety and efficacy of drops to treat presbyopia.
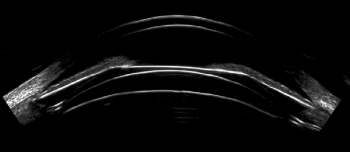
Advancements in imaging make ICL an effective option for a wider range of patients.

Ophthalmologists at New York Eye and Ear Infirmary of Mount Sinai have created a new technique to evaluate patients with sickle cell retinopathy and assess the disease before it progresses and leads to permanent vision loss.

In an announcement that could impact ophthalmologists, the Department of Health and Human Services’ Office for Civil Rights revealed this week that it will enforce a prohibition of discrimination on the basis of sexual orientation and gender identity.
Oculis reported positive data from two of its clinical proof-of-concept phase 2 trials for OCS-02, a novel topical anti-TNF alpha antibody fragment candidate, for the treatment of dry eye disease and acute anterior uveitis.

Graybug Vision’s Phase 2b ALTISSIMO trial of GB-102 focuses on the treatment of wet age-related macular degeneration.
Findings from retrospective study support intraoperative cefuroxime irrigation.

Joseph Grieco, PhD, notes that treatment fills a gap for patients who are unresponsive to the standard treatments for noninfectious keratitis.

Ocular Innovations debuts an option that delivers content touchpoints through a frictionless mobile experience.

Tarsus Pharmaceuticals kicks off Saturn-2, trial, which is designed similar to Saturn-1, first pivotal trial for TP-03. Topline data from Saturn-1 is expected in July.