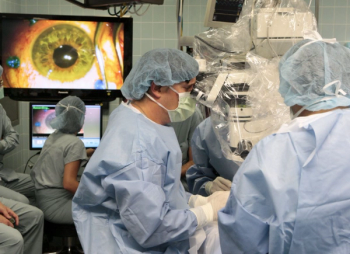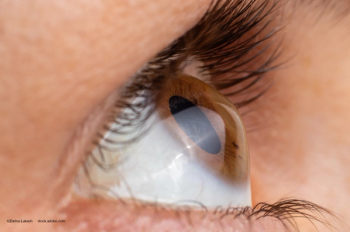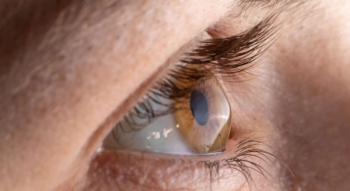
The company’s iLink therapy for the treatment of keratoconus consists of novel single-use drug formulations that are bio-activated by proprietary systems through the delivery of ultraviolet light to the cornea to induce corneal cross-linking designed to strengthen, stabilize and reshape the cornea.