
The country’s Ministry of Health has issued infringement notices to two ophthalmologist for seeing patients while unvaccinated.

The country’s Ministry of Health has issued infringement notices to two ophthalmologist for seeing patients while unvaccinated.
The MIRA-3 Phase 3 FDA Registration trial for Nyxol met its primary endpoint with 58% of treated subjects returning to baseline pupil diameter at 90 minutes compared to 6% of placebo subjects.
The company’s iCare HOME2 self-tonometer allows patients to collect real-world IOP data at any time.

The EVO/EVO+ Visian Implantable Collamer Lens provides visual freedom independent of contact lenses and glasses.

The San Francisco-based company also announces that Raj K. Maturi, MD, is the first PI in the network.
Vanderbilt’s David Calkins, PhD, was honored with the 2022 President’s Award, presented by the Glaucoma Research Foundation, at the Glaucoma 360 Annual Gala last month.

Investigators theorize that the ophthalmic nerve may be affected by the COVID-19 virus, and therefore may affect the corneal esthesiometry values.

Lower doses vs higher ones are equally effective for treating disease

Investigators have questioned the potential for the vascular density to be a biomarker for microvascular abnormalities following COVID-19 and found that longitudinal studies are needed to determine this.

According to the company, VVN001 demonstrated clinical and statistical superiority over vehicle in reducing total and sub-regional corneal fluorescein staining scores.

Raj Kannan, the new CEO of Aerie Pharmaceuticals, talks with Ophthalmology Times' David Hutton about what's coming down the pipeline for Aerie.
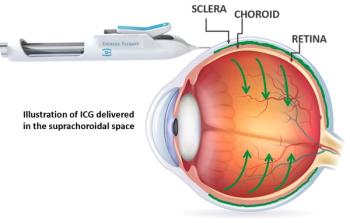
Provides safe, effective injection to target choroidal, retinal tissues
According to a team of investigators at University of California, Berkeley, tests of the drug Antabuse could prove the role of hyperactive retinal cells in blindness, potentially leading to better therapies.
Drug is prescribed as adjunctive therapy for those with glaucoma

Gary Jerkins, MD, a Nashville physician who founded SeaStar Kids in 2010 to help disadvantaged Ukrainian youth, has made 47 trips to Ukraine since 1996 and has hope for its future.

"Tell Me Your Secrets" is the theme of the virtual April 2 event, which is expected to build on the foundation established during the first program held last fall.
Physicians offer pearls to improve visualization in eyes using retroillumination techniques
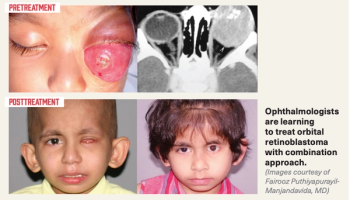
Investigators observe survival rate with orbital retinoblastoma improves substantially due to a combination approach that includes intensive sequential treatment comprised of chemotherapy, enucleation, and external beam radiation therapy.

When it comes to the sexes in the operating room, the key is collaboration, with a goal of presenting patient suffering.
Short-term steroid use can alleviate patient discomfort.
The injection of dexamethasone intraocular suspension 9% into the capsular bag may change the standard of care following cataract surgery.

While investigators have found a link between dry eye and depression, the exact reasons behind the link are not immediately known.

According to the company, pegcetacoplan demonstrated continuous and clinically meaningful effects at month 18 in the studies, which also found that treatment effects in DERBY were comparable to OAKS during months 6 to 18. The combined 18-month data show the potential for improving treatment effects over.

Anton Vlasov, DO, moved to the United States in 2001 at the age of 14, but since Russia invaded his homeland last month, the safety of family still living in Ukraine has weighed heavy on his mind.
Physicians find the Vivity lens offers their patients reduced glare, halos and increased spectacle independence.

The study, conducted with Tufts Medical Center, represents a breakthrough showing that Microdose Array Print technology can provide similar benefits of non-preserved medications.
The key factors include type, case complexity, and history of diabetic retinopathy/proliferative diabetic retinopathy.
Dr. Rishi Raj Borah, Country Director with Orbis India, discusses recent research he co-authored showing the top 3 barriers keeping families from accessing cataract treatment for their children in India.

The latest announcement comes after the previously granted ODD for polihexanide in acanthamoeba keratitis by the FDA and EMA.
Mary Durbin, PhD, Chief Scientific Officer at Heru, discusses the benefits and capabilities of the various testing modalities available in 1 wearable platform.