
Ferdinand-Braun-Institute in Berlin, Germany is developing a solution with its semiconductor-based laser sources.

Ferdinand-Braun-Institute in Berlin, Germany is developing a solution with its semiconductor-based laser sources.

Nicole Bajic, MD, and Neel S. Vaidya, MD, MPH, share their insights from when they were at this crossroads. Learn more clinical pearls on topics like this and more at Real World Ophthalmology’s virtual conference, “Top 10 Things I Wish I Knew Sooner,” this Saturday, November 5.
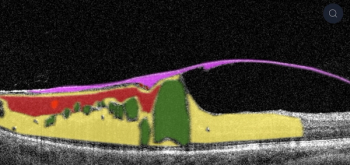
The company's AI cloud-based platform is available via a browser to any eye care professional in the world. The artificial intelligence algorithm was trained on millions of graphically labeled OCT scans collected from ophthalmic clinics.

The study is one of the first clinical trials to validate the clinical utility of real-world remote physiologic monitoring using an on-demand vision-as-a-service extension to the specialty retina clinic to enable remote monitoring of patients with chronic diseases such as AMD, diabetic retinopathy, and other retinal conditions.

Lawrence Whittle, Chief Commercial Officer for Verana Health, explains what the significance of imaging data linked with electronic health record data means for patient outcomes.
Nicox reports that daily dosing of NCX 470 0.1% met the primary efficacy objective of demonstrating non-inferiority to latanoprost 0.005%.

Replay is launching Eudora, an HSV gene therapy company that will target genetic retinal diseases.

Prevent Blindness offers videos, fact sheets, social media graphics and PowerPoint presentations to help ophthalmologists educate the public on the potential effects diabetes may have on vision.

Scott Walter, MD, an investigator in the TENAYA and LUCERNE trials discusses the year 2 data for the treat-and-extend regimen of faricimab for the treatment of nAMD and DME.

The EyeBuddy app will bring telehealth to eye doctors in the coming months, connecting patients across Bangladesh with ophthalmologists at a major eye-care hospital.

Marc Berger, Chief Operating Officer at Verana Health, highlights what a new initiative with the American Academy of Ophthalmology IRIS Registry will mean for patients and physicians.

Financial experts point out that what you don’t know you don’t know can hinder you.

The FDA has set a PDUFA goal date of August 29, 2023. Bevacizumab-vikg, if approved, is expected to receive 12 years of regulatory exclusivity in the United States.
A previously uncharacterized area of the protein known as RPE65 spontaneously turns spiral-shaped when it encounters intracellular membranes, or thin structures that surround different parts of a cell.

CEO Rob Thornhill discusses the features of the device, as well as plans for a new technology called ZEPTOLINK which combines the company’s novel ZEPTO technology with any phaco device.

The 10-year retrospective ALOFT study details the importance of digital remote monitoring for patients diagnosed with age-related macular degeneration.

Teprotumumab-trbw is the first and only medicine approved by the FDA for the treatment of thyroid eye disease.
Complimentary registration is open for this unique meeting designed to prepare young ophthalmologists for success in early practice.

There literally are not enough hours in a day to allow physicians to comply with all the recommendations emanating from the various constituencies involved in issuing guidelines.

Vivek Patel, MD, discusses issues that could threaten sight during presentation at the American Academy of Ophthalmology annual meeting in Chicago.

Ula V. Jurkunas, MD, discusses a presentation she gave recently at the American Academy of Ophthalmology’s 2022 annual meeting in Chicago on the use of limbal stem cells to treat corneal blindness.
The COVID-19 pandemic has led to greater comfort with robotic telepresence in eye screenings.

According to a study, health disparities impact the rates of pediatric ophthalmology visits.

An interactive agenda at the Ophthalmology Times® EyeCon 2022 at the JW Marriott Marco Island Beach Resort in Florida will foster an open exchange of ideas with fellow attendees from across the United States.

According to Mark Packer, MD, FACS, observations include key data points and clinical implications.

At AAO, Kamran Riaz, MD, discussed the new textbook, "Optics for the New Millennium," which is intended to be a compendium resource for ophthalmologists.

LENZ Therapeutics has unveiled positive topline data from its INSIGHT study achieved by 2 formulations to treat presbyopia.

Harvey Uy, MD, has been using the Ally adaptive cataract treatment system at his clinic in the Philippines, and it is allowing him to be more efficient.

Lisa M. Nijm, MD, JD, shares highlights from the Real World Ophthalmology 'After Dark' networking reception held at the American Academy of Ophthalmology annual meeting. The next Real World Ophthalmology meeting on November 5—which will be virtual—will cover important clinical, business, and personal growth areas for young ophthalmologists.

According to a BBC News report, the woman showered while wearing her lenses and she contracted a rare parasite infection called Acanthamoeba keratitis.