
CXL has been proven effective to treat patients with corneal ectasias.

The results represent a major step towards first in-human clinical studies for this program - the first potential therapy for patients with geographic atrophy secondary to AMD that addresses the underlying causes of the disease.

Receiving FDA orphan drug designation allows the company to proceed with plans to advance the development of BRM424, a novel, first-in-class, potential treatment for neurotrophic keratitis.

The Ernest E. Tschannen Eye Institute is dedicated to eye care and sight restoration through technology, pioneering research and eye care clinicians.

According to the company, the NDA is supported by positive phase data demonstrating the rapid reversal of dilated eyes and favorable safety profile in pediatric and adult subjects.

Researchers from the Deutsches Zentrum für Neurodegenerative Erkrankungen and the Center for Regenerative Therapies Dresden at TU Dresden have found that visual cells in the human retina may not simply die in some diseases, but are mechanically transported out of the retina beforehand.
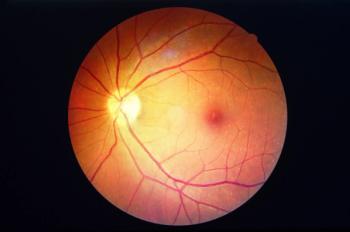
According to researchers, cells within retinal blood vessels are endowed with a previously unappreciated ability to acquire resistance against the damaging effects of hyperglycemia in patients with diabetes mellitus.

SPVN06 is a breakthrough gene therapy approach aimed at stopping or slowing disease progression in patients affected by IRDs and dry age-related macular degeneration (AMD), regardless of their genetic background.

Weigh in on this month's survey question. The poll will be open until December 16, 2022.

OPGx-001 is Opus’ first program to enter clinical evaluation and is designed to address vision loss due to mutations in the LCA5 gene, which causes one of the most severe forms of early-onset blinding disease Leber congenital amaurosis.
In an effort to educate the public on GA, Prevent Blindness has created a variety of resources for patients in English and Spanish.

Aflibercept and bevacizumab are the two most frequently used therapies for the treatment of wet AMD.

According to the company, the facility will serve as a hub of the EVO Lens-Based Experience for ophthalmic surgeons, staff and healthcare practitioners.

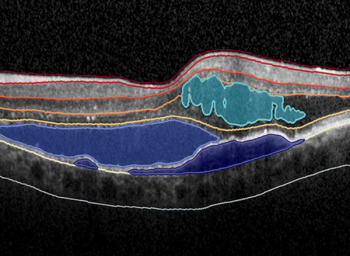
Discovery CORE and its AI models have been designed to accelerate data analysis and help clinical and academic researchers collaborate more efficiently in real time with their peers on medical and imaging datasets.

In a two-decade career as a researcher at Johnson & Johnson Surgical Vision, Piers has been a part of teams at the forefront of a number of innovations in IOLs. She discusses her career and the outlook for research and development in ophthalmology.
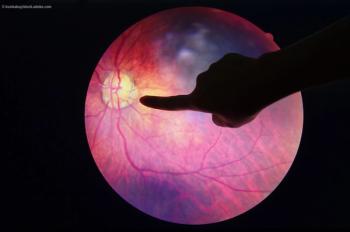
Despite the latest advances in anti-VEGF therapy, a small group of patients do not achieve improved vision. Ophthalmologists are looking for options.

According to physicians, patients who do not undergo second procedure are more likely to develop glaucoma.

Re-Vana has demonstrated that its platform can achieve 6 months or greater sustained delivery of an anti-VEGF biologic at therapeutically-relevant levels with high drug loading, controlled burst release, and a favorable degradation profile.

The deal will provide Harrow’s entire formulary compounded ophthalmic products to iOR Partners’ expanding office-based surgery locations across the United States.

The groups invite those attending the annual conference to join them at their event on Monday, January 16, which is free to OWL and WIO members.
According to investigators, the risk for the development of dry eye disease is greater in postmenopausal women compared with those who have not reached menopause as a result of hormonal dysregulation of the ocular secretory glands.
Investigators who are examining end-stage disease see significant visual recovery in 2 patients.

Patients surveyed say the handheld tool proved to be comfortable and easy to use.

The therapy offers improved retinal sensitivity, visual function, as well as improvements in mobility.

Investigators who are examining end-stage disease see significant visual recovery in 2 patients.

The SBL-3 ClearView clinical study, an FDA investigational device exemption clinical trial conducted at 18 sites across the US, is a prospective, subject-masked, randomized, two-arm study.

The deal adds pharmaceutical research and development capabilities and further expertise for future product pipeline. It also expands Alcon’s presence in the $20 billion global ophthalmic pharmaceutical category.

A recent study showed that only 6% of practicing ophthalmologists in the US identify as an underrepresented minority.

Oculis SA has published positive data of its licaminlimab active-controlled, multicenter, randomized, parallel-group Phase 2 clinical trial assessing the effect of topical licaminlimab on anterior chamber (AC) cell grade in patients with acute anterior uveitis.