Refractive Surgery
Latest News
Latest Videos

CME Content
More News

Ophthalmologists discuss topography-guided techniques, long-term corneal health, and emerging technologies set to transform refractive surgery.
Automated standardized graphs facilitate refractive surgery outcome analysis.
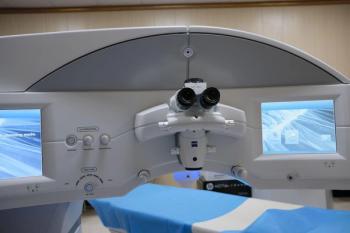
Faster cutting speeds and ergonomic design streamline workflows and enhance comfort.
Explore groundbreaking advancements in eye care from the 2000s, including LASIK innovations, anti-VEGF treatments, and enhanced imaging technologies.

Explore the groundbreaking advancements in ophthalmic technology from the 1980s and 1990s, including excimer lasers, LASIK, and innovative imaging techniques.

A young surgeon reflects on training, technology, and the human side of adopting new excimer laser platforms.

Both have issued further statements regarding the deal.

Wavelight plus was first launched in China, with several European and Asia-Pacific markets following suit

Opus Genetics doses first patient in LYNX-3, phase 3 trial of phentolamine ophthalmic solution 0.75%
Phentolamine ophthalmic solution 0.75% is for the treatment of significant, chronic night driving impairment in keratorefractive patients with reduced mesopic vision.

Innovation, adaptability, and clear communication are key to managing outcomes.

Marguerite B. McDonald, MD, FACS, shares a front-row view of ophthalmology’s “revolution with a capital R."

Careful management of patient expectations is key to achieving optimal outcomes.

Ophthalmologists discuss the most impactful advancements reshaping patient care over five decades.

In honor of Ophthalmology Times’ 50th anniversary, anterior segment surgeons attending ASCRS 2025 weigh in on the innovations that defined modern ophthalmology.
Lupin Limited secures FDA approval for Loteprednol Etabonate Ophthalmic Gel, enhancing treatment options for postoperative ocular inflammation and pain.
Q&A: Kira Manusis, MD, on how the Center for Refractive Solutions is redefining patient care at NYEE
As its director, Manusis outlines the facility’s role in delivering advanced, personalized solutions to improve vision and patient experience.

The center aims to optimize patients' vision with a range of cataract and corneal refractive procedures and minimize reliance on glasses and contacts.

Opus Genetics releases topline results from LYNX-2 evaluating phentolamine ophthalmic solution 0.75%
The phase 3 trial observed the treatment of chronic night driving impairment in keratorefractive patients with reduced mesopic vision.

George O. Waring IV, MD, FACS, discusses the “vision for a lifetime” approach and how modern advancements allow tailored treatments for every stage of eye maturity.

Majmudar encourages young surgeons to seek hands-on experience early and often, stressing the importance of real-world practice beyond the classroom.

For patients with high myopia, an implantable ICL offers certain advantages over LASIK or other traditional refractive surgeries, Dr. Kim said.
Mah previews a packed 2025 Annual Meeting, from the debut of SightLine to Dr. Glaucomflecken’s return to the main stage, and outlines his priorities for the year ahead as incoming ASCRS president.

The May 4 event, themed around Star Wars, will feature the latest innovations, foster open discussions, and provide a collaborative environment for networking and learning, with sessions covering IOLs, oculoplastics, glaucoma, retina therapy, and refractive surgical options.
Vision correction has evolved into a continuum of procedures suited for different stages of ocular maturity