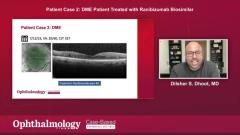
Patient case 2: DME Patient Treated with Ranibizumab Biosimilar
Dilsher S. Dhoot, MD discusses a recent case-based roundtable meeting on anti-VEGF biosimilars, highlighting the first of two case discussions.
Episodes in this series
Summary
Dilsher Dhoot presented a second case at the round table, focusing on diabetic macular edema (DME). The patient, a 76-year-old female, had received previous injections of ranibizumab but was lost to follow-up. Upon reevaluation, she presented with vision issues and typical diabetic findings.
The roundtable discussion delved into physicians' comfort levels prescribing biosimilars for age-related macular degeneration (AMD) and DME, with the majority expressing confidence in doing so. Dhoot emphasized his practice's use of biosimilars for AMD, similar to how they would use the reference product.
Dhoot highlighted that biosimilars provide valuable treatment options, potentially offering cost savings for both the healthcare system and patients. He emphasized their role in providing effective and beneficial treatments for patients with conditions like AMD and DME, ultimately improving visual acuity over the long term.
This summary was AI-generated and edited for clarity.
Newsletter
Don’t miss out—get Ophthalmology Times updates on the latest clinical advancements and expert interviews, straight to your inbox.