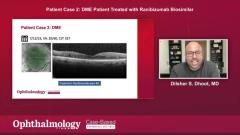
Patient case 1: Neovascular AMD Patient Treated with Ranibizumab Biosimilar
Dilsher S. Dhoot, MD discusses a recent case-based roundtable meeting on anti-VEGF biosimilars, highlighting the first of two case discussions.
Episodes in this series
Summary
Dilsher Dhoot, MD, a clinician from Retina Consultants in Santa Barbara, California, recently moderated a roundtable discussion on biosimilars for Ophthalmology Times™. The participants were a diverse group of physicians from around the country, with varying levels of familiarity with biosimilars. Some had used them extensively, while others were less experienced and had concerns about reimbursement and clinic logistics.
Dhoot, MD presented a case of a 73-year-old patient who initially responded well to treatment but later converted to neovascular age-related macular degeneration. The patient showed a positive response to a biosimilar, with improved vision and stable results over multiple injections.
Regarding concerns with prescribing approved biosimilars, most physicians had confidence in the rigorous development process. However, there were considerations for patients requiring more potent or durable drugs, as switching to a biosimilar might be viewed as a step down.
This summary was AI-generated and edited for clarity.
Newsletter
Don’t miss out—get Ophthalmology Times updates on the latest clinical advancements and expert interviews, straight to your inbox.