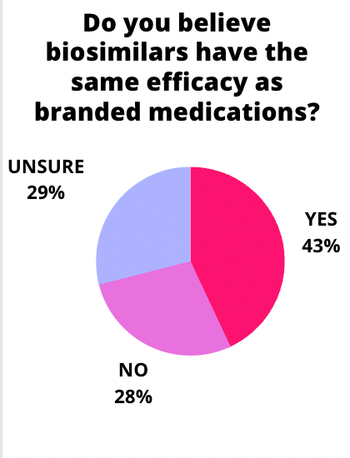
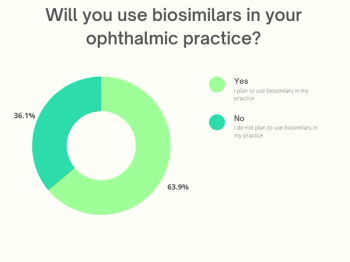
Samsung Bioepsis' SB15 was comparable to Eylea (aflibercept) in a 56-week randomized clinical trial that included a rerandomization at 32 weeks. The rerandomization resulted in 111 study volunteers switching from Eylea to the biosimilar for the last part of the study.