
Language can drive understanding, trust, and better outcomes for patients

Language can drive understanding, trust, and better outcomes for patients

According to the company, it is anticipating cost savings of up to $300 million through 2024, which includes an estimated 25% reduction in current workforce and a reduction in external expenses.

ATSN-201 leverages novel spreading capsid to overcome challenges associated with intravitreally delivered AAVs in the treatment of XLRS.

TOUR006 is an anti-IL-6 antibody with a differentiated profile for the treatment of thyroid eye disease (TED).
The organization says to stop using Dr. Berne’s and LightEyez MSM drops immediately due to bacterial contamination, fungal contamination, or both.
More than 135 patients have been recruited in 4 months for Phase 2 study of a single intravitreal (IVT) injection of ONL1204 ophthalmic solution as an adjunct to standard-of-care surgery.

For clinicians, the evolution of treatment is as much a responsibility as an opportunity.

The investigators performed a retrospective cohort study of patients from 10 US ocular inflammatory disease (OID) subspecialty practices.

Amber Bio is developing a first-of-its-kind RNA writing platform using multi-kilobase edits to reach previously undruggable patient populations.

Treating the meibomian glands and lifestyle changes are among some of the treatment methods.

The research by Scott N J Watamaniuk, PhD, has potential implications for patients diagnosed with strabismus.

Take a look at a review of the highlights and hottest stories from Ophthalmology Times during the week of August 20, 2023.

Eva Kim, MD, spoke with our team to share insights from her presentation on ICL versus CLE at the Women in Ophthalmology Summer Symposium 2023 in Marco Island, Florida.

Currently, miotics are taking center stage for treating presbyopia.


Henriquez pointed out that waiting for keratoconic progression runs the inherent risk that keratoconus will progress rapidly and affect the planned initial treatment protocol.
The study, conducted by the National Eye Institute and University of Freiberg, Germany, sheds light on nerve function in the brain and retina.

Lead author Sean Paul, MD, reports radiofrequency treatment in conjunction with expression of the meibomian glands reduces the signs and symptoms of dry eye disease.
One practice’s experience with office-based surgery validates peer-reviewed study.
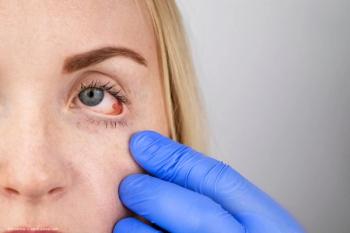
TRS01 demonstrates clear anti-inflammatory activity in noninfectious anterior uveitis with no evidence of significant adverse effects.
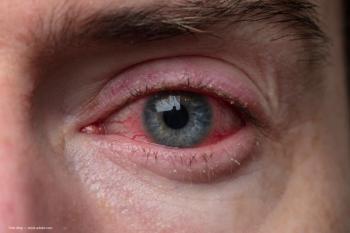
Lotilaner ophthalmic solution 0.25% (Xdemvy) was approved by the FDA in July, making it the first and only approved treatment for Demodex blepharitis.
The company believes the 19-gauge filter needles may be the culprit in the SYFOVRE injections.

This intellectual property protects Kiora's pipeline by covering multiple small molecule analogs, delivery methods, and several inflammatory-related therapeutic applications, including posterior non-infectious uveitis, extending market exclusivity for approved indications in the United States and Europe.

A team of researchers have successfully transplanted human microglia into a mouse retina to create a model for studying eye disease treatments, such as diabetic retinopathy, glaucoma and age-related macular degeneration.
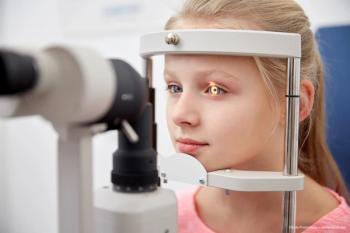
According to researchers from University of Michigan and Duke University, children with Medicaid or other public insurance are far less likely than those with private insurance to have had a vision check in the last year at a primary care office.

Researchers have identified markers that indicate the presence of Parkinson’s disease in patients an average of 7 years before the disease presents clinically.

Clinicians' expertise can help assess inconclusive findings about the benefits of blue-light filtering glasses. This enables them to offer informed guidance to patients concerned about eye strain and sleep quality.

The company noted that its Phase Ib dose-escalation clinical trial will evaluate safety, tolerability and efficacy of VG901 in patients with RP due to biallelic CNGA1 mutations.
Cultivated autologous limbal epithelial cells (CALEC) procedure shown to be safe and feasible with early positive results of restored cornea surfaces or vision gains in four patients with severe chemical burns.

According to the company, the primary endpoint for the NEXPEDE-1 study of lufepirsen ophthalmic gel will be complete corneal healing as determined by corneal fluorescein staining.