According to data presented at the American Academy of Ophthalmology annual meeting in San Francisco, a study of children with cell disease finds 1 in 3 had retinopathy.

According to data presented at the American Academy of Ophthalmology annual meeting in San Francisco, a study of children with cell disease finds 1 in 3 had retinopathy.

Edward J. Holland, MD, and Professor Shigeru Kinoshita, MD, discuss how a single donor cornea could potentially help over a thousand patients.

Results showed that IZERVAY met the primary objective of reducing the rate of geographic atrophy (GA) growth in patients treated monthly compared to sham-treated patients

David Hutton of Ophthalmology Times spent time with Esen K Akpek, MD, who discussed her presentation at this year's American Academy of Ophthalmology meeting.

Kerrie Brady, president and chief executive officer of OcuTerra Therapeutics, shares progress in their Phase 2 DR:EAM Diabetic Retinopathy study, with promising results expected in Q1 2024 for their innovative eye drop treatment, OTT166.

The American Academy of Ophthalmology (AAO) 2023 annual meeting is returning home to San Francisco on Friday November 3 through Monday November 6.

Ayush Parikh, MD, and colleagues conducted a retrospective review of and found that spontaneous closure and surgical treatment yielded comparable rates of closure and visual outcomes.

The latest innovations are in focus at the 127th annual meeting of the American Academy of Ophthalmology, being held at the Moscone Center in San Francisco.

Launched in 2014, Intelligent Research in Sight is the nation's first and largest comprehensive eye disease registry.
Researchers are addressing growing concern over vision loss in people taking semaglutideat the American Academy of Ophthalmology annual meeting in San Francisco.

The collaboration, fueled by a $5 million donation from Knights Templar Eye Foundation (KTEF), will introduce a free and open VR simulation program for ophthalmologists and trainees worldwide.
The purpose of this investigation was 2-fold: to estimate the costs of treating GA with pegcetacoplan and to identify possible utility measures to compare treatments for GA.

The goal is to evaluate neonates with retinopathy of prematurity (ROP) who met only 1 screening criterion with the goal of easing the burden of ROP screening.

Eight patients were able to receive a second implant at 21 weeks, with no product related adverse effects.
The company’s AI CLAIR technology platform has received FDA breakthrough device status, and if cleared by the FDA will deliver real-time cardiovascular disease risk assessments through routine eye exams.

Researchers conducted a study on 145 patients with night vision problems, testing the effects of 0.75% phentolamine ophthalmic solution. The trial demonstrated substantial improvement in visual acuity on both evaluation days.

OCT and OCTA imaging were especially valuable in this patient population because the images extended the current definition of sickle cell retinopathy.

Light-adjustable lens in patients with previous RK: Improved visual and refractive outcomes after cataract surgery.

The clinical trial will evaluate the safety and efficacy of CLS-AX (axitinib injectable suspension), a tyrosine kinase inhibitor. According to the company, topline data is expected during the third quarter of 2024.
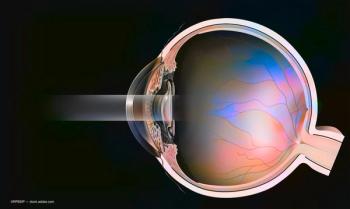
Physician notes endothelial cells play key role in hydration, transparency of cornea.

According to the company, its Phase I/II trial is being initiated to investigate SpyGlass platform for the long-term delivery of bimatoprost in patients with glaucoma and visually significant cataracts.

By taking a proactive approach to treatment, ophthalmologists can identify the “silent sufferers.”

New drugs are now in the pipeline to focus on treatment of the disease.

According to the company, its ViaLase Laser combines the precision of femtosecond laser technology and the accuracy of micron-level image guidance to deliver the world's first femtosecond laser image-guided high-precision trabeculotomy.

Patients who have signs or symptoms of an eye infection after using these products should talk to their health care provider or seek medical care immediately.
With the combined system, surgeons will also save space in the operating room and optimize cataract surgery all on one system.
Acanthamoeba keratitis can lead to inflammation of the cornea and result in extreme levels of pain, light sensitivity and vision loss.

Prevent Blindness is offering videos, fact sheets, social media graphics and PowerPoint presentations to educate the public on the potential effects diabetes may have on vision.

Transparent pricing in elective surgeries is crucial.

When patients pay for elective procedures, premium experience often falters.