
According to the companies, the merger creates a NASDAQ-listed, late clinical-stage biopharmaceutical company focused on advancing LENZ Therapeutics’ lead assets for the treatment of presbyopia.

According to the companies, the merger creates a NASDAQ-listed, late clinical-stage biopharmaceutical company focused on advancing LENZ Therapeutics’ lead assets for the treatment of presbyopia.

Loretta Alborghetti, from Redditch, UK, a medical secretary within the ophthalmology department at Worcestershire Acute Hospitals NHS Trust, illegally accessed the records of more than 150 patients.

Charles Wykoff, MD, PhD, spoke with Ophthalmology Times about the GALE study data at one year looking at pegcetacoplan for the management of geographic atrophy at this year's American Academy of Ophthalmology meeting.

Approach to lowering IOP reduces medication burden without risking graft rejection.

Researchers found reduced iron concentration in the clear gel part of the eye of human patients and iron accumulation in the retina of mice.

The improvement of accessibility and education could drive changes in outcomes.

This is just one of many times the FDA has issued a warning to companies for the sale of unapproved ophthalmic products in 2023.

Procedure can reduce scleral biomechanical stiffness and rejuvenate dynamic range of focus.

Mariam Maghribi, Chief Business Officer for Shifamed, spoke with Ophthalmology Times about the Atia Vision IOL device at this year's American Academy of Ophthalmology meeting.

ATSN-101, a gene therapy for GUCY2D-associated Leber congenital amaurosis, has demonstrated clinically meaningful improvements in vision at the highest dose with no drug-related serious adverse events 6 months post-treatment in ongoing Phase I/II clinical trial.
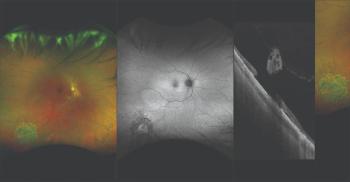
Technology is an option for evaluating, documenting certain types of retinal pathology.

Nick Curtis, CEO at LENSAR, met with Ophthalmology Times to discuss their co-development with Oertli on their phaco device and LENSAR's Ally treatment system at the American Academy of Ophthalmology.

Previously, the EMA had approved Eylea in a dosage of 2 mg. After considering the EMA’s recommendation, the European Commission will decide whether to issue final approval of the drug.

Peter J. McDonnell, MD, and Neda Shamie, MD, consider the transformative landscape of cataract surgery, highlighting the impact of small-aperture IOL technology. The conversation delves into its potential benefits, influence on the market, and the evolving role of cataract surgeons in guiding patients through advanced options.

The latest in Optic Relief from Ophthalmology Times
TRS01 has the potential to be the first-line treatment option for non-infectious uveitis and specifically uveitic glaucoma, according to the company.


Rick Lewis, MD, Chief Medical Officer for ViaLase met with Ophthalmology Times to discuss completion of their important pivotal trial called VIA-002, which is a randomized prospective trial of the use of the ViaLase Laser versus SLT in adult patients with primary open angle glaucoma at the American Academy of Ophthalmology.

The week focuses on bringing awareness to TED through emphasis on mental health and art therapy solutions.

Penny Asbell, MD, FACS, spoke with Ophthalmology Times about her poster on results from the ARMOR Study and latest diagnostic and therapeutic advancements in ocular infections at this year's American Academy of Ophthalmology meeting.

Sean Hanlon, CEO of CoFi, spoke with Ophthalmology Times about company updates as well as their integration with CareCredit at this year's American Academy of Ophthalmology meeting.

Researchers found the growth of RPE cells was successfully controlled by the stencil patterns. When RPE cells were not held together correctly, the cells excreted levels of harmful proteins that could contribute to vision loss.

According to the company, OCU410ST uses an AAV delivery platform for the retinal delivery of the RORA (RAR Related Orphan Receptor A) gene.

Clara Chan, MD, FRSCS, FACS, DABO, from the University of Toronto spoke with Ophthalmology Times about their study on the consistency of outcomes across 3 different cohorts of patients that were internationally collected at this year's American Academy of Ophthalmology meeting.

Oliver Hvidt, CEO and co-founder of Norlase, spoke with Ophthalmology Times about the company's 4-year journey from AAO 2019 to present at this year's American Academy of Ophthalmology meeting.

Jayashree Kalpathy-Cramer, PhD, was granted $300,000 from the Michael J Fox Foundation to examine key data put together at the Sue Anschutz-Rodgers Eye Center with artificial intelligence in an effort to identify biomarkers of Parkinson’s disease.

Swarup Swaminathan, MD, spoke with Ophthalmology Times about using the Heidelberg SPECTRALIS and caring for our Glaucoma patients at this year's American Academy of Ophthalmology meeting.

Daniel Chang, MD, spoke with Ophthalmology Times about his poster "Clinical performance of a diffractive, extended depth-of-focus IOL, with violet light filtering and high-resolution lathing" at this year's American Academy of Ophthalmology meeting.

Researchers from Anglia Ruskin University and the University of Oxford conducted a study that reveals knowledge gaps for conditions that affect 18-64-year-olds in the UK.