In an annual report, the company revealed the impact of a dramatic increase in commercially available biosimilars and progress in the areas of patient accessibility and treatment affordability.

In an annual report, the company revealed the impact of a dramatic increase in commercially available biosimilars and progress in the areas of patient accessibility and treatment affordability.
Stargardt disease is an orphan blindness disease that affects approximately 35,000 people in the United States.

According to the company, its proprietary non-viral gene therapy platform with minimally invasive delivery technology is providing long lasting gene expression and favorable distribution in the retina.

Bonnie An Henderson, MD, leads HelpMeSee to provide advanced cataract surgery training globally, emphasizing humanitarian aid and innovative simulation technology.

Several eye care products sold at Walmart and CVS locations have been recalled due to FDA findings during a facility inspection conducted earlier this month.
According to the company, 2,500 clinical and peer-reviewed studies that span 235 disease states have demonstrated the clinical utility of Optos technology.

The patch is the world's first non-degradable, synthetic tissue substitute for ophthalmic surgery.
ECP105 has been developed for the recovery of post-surgical treatment of glaucoma.

Prevent Blindness is offering a variety of free educational resources to the public and professionals

Researchers have found that the interactive release of anti-inflammatory drugs depend on the level of retinal degeneration. A customized treatment approach is expected to be developed to reduce patients’ inconvenience of having multiple shots.
Structural and vascular changes detected in RVO-affected eyes and unaffected fellow eyes
Increased levels of p-tau181 and p-tau127 and a decreasing cerebrospinal fluid Aβ42/40 ratio better defines progression from preclinical to manifest Alzheimer’s disease.

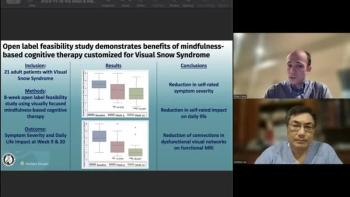
AI matched and outshone human specialists.

Annual meeting continues to offer key education opportunities.

The company will add Pravin U. Dugel, MD, as executive director, and add Jeffrey S. Heier, MD, Peter K. Kaiser, MD, and Sanjay Nayak, PhD, MBBS, to its management team.

The platform streamlines clinical decision workflows, management of teleophthalmology, referral decisions and patient management while improving clinic efficiencies.
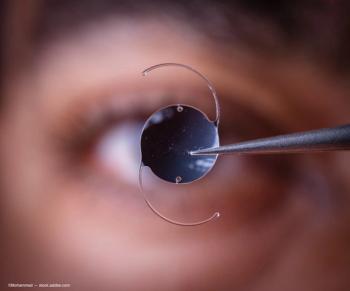
The implantable lenses can offer an advantage over laser surgery.

Neda Shamie, MD, highlights the origins and growth of CIME and what makes it unique from other meetings.

Physicians offer pearls for the treatment of patients diagnosed with these diseases
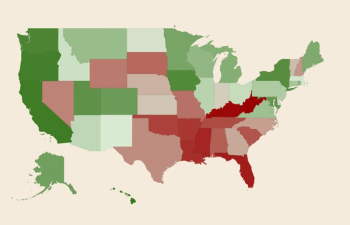
RX Safety conducted an analysis across all 50 US states and Washington DC to determine which state has the best eye health, as well as the worst.

Corneal endothelial cells from a single donor tissue could be amplified using Emmecell’s technology to restore vision for hundreds of patients via injectable cell therapy.

The trial of esonadogene mvoparvovec (Opvika) will take place in the US for the treatment of Leber hereditary optic neuropathy caused by ND4 mutation (ND4-LHON).

A new study shows that much of an older person with visual impairment's physical activity is achieved at home. Improved home lighting can help increase those at-home activity rates.

Cutting-edge advances are outlined at Hawaiian Eye and Retina 2024

Early keratoconus screening advocated

Rajendra Apte, MD, PhD, has received the Research to Prevent Blindness /American Macular Degeneration Foundation Catalyst Award for innovative research approaches in studying age-related macular degeneration.

Teaching fellows can prove to be a rewarding experience for instructors.

Products under the terms of the agreement include VERKAZIA, Cationorm PLUS, VEVYE, IHEEZO, and ZERVIATE.