The 1-time, intracameral injection is a cell therapy for the treatment of corneal edema secondary to corneal endothelial dysfunction.

The 1-time, intracameral injection is a cell therapy for the treatment of corneal edema secondary to corneal endothelial dysfunction.

Following the CRL from the FDA last year, Aldeyra is planning to initiate a dry eye chamber clinical trial in the first half of 2024.

Ocular toxicity from breast cancer chemotherapy, head and neck cancer radiation and targeted therapy.

The Bascom Palmer Eye Institute at the University of Miami Miller School of Medicine recently received a $1 million donation to drive its efforts to successfully transplant a whole eye.

The company announced it has entered into a global licensing agreement with ForSeeCon Eye Corp. for the company's ophthalmology pipeline, which includes the medical device Vitargus.
Kay Durairaj, MD, FACS, known as Dr Kay, discusses blepharoplasty and the collaboration between facial plastic surgeons and ophthalmologists in a conversation with Ophthalmology Times.

Since its U.S. launch in September 2023, more than 40,000 avacincaptad pegol intravitreal solution vials have been distributed to physician practices.

According to Alcon, as CMO, Terry Kim, MD, will continue to integrate scientific and clinical priorities across Alcon’s Surgical and Vision Care franchises, with a focus on meeting the needs of patients and eye care practitioners across the globe.

The NOVA clinical trial has found statistical non-inferiority to current clinical standards for sensitivities at individual test locations, glaucoma staging using Medicare definitions, and an exceptionally high correlation of 0.94 in mean deviation.

Stargardt disease is the most common inherited retinal dystrophy (causing blurring or loss of central vision) in both adults and children.

The new cohort will add patients living with IRDs caused by mutations in multiple genes to the already existing cohort of patients with IRDs caused by single gene mutations.

The positive opinion concerns authorization of bevacizumab gamma (ONS-5010/LYTENAVA), a formulation of bevacizumab for treatment of wet age-related macular degeneration (wet AMD).

Conference defines the future of anterior segment surgery.

Alice Epitropoulos, MD, discusses the importance of considering ocular surface health in cataract surgery patients.

A Purdue University researcher is leading a team that is creating patent-pending smart contacts for glaucoma, neovascularization and dry eyes.

In a recent study led by Steven Pittler, PhD, and his team at the University of Alabama Birmingham (UAB), the role of modifier genes in retinitis pigmentosa type 59 (RP59) was meticulously examined.

Results from the trial by Nannodropper show microdrops could reduce waste, cost, and side effects while being as effective as traditional size drops.

The prevalence of AMD features in Republic of Korea Air Force pilots was higher than in other general populations studied.

Multiple symptomatic improvements, as well as tear film break-up time improvements and a reduction in ocular pain, were noted.
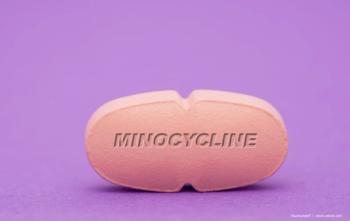
The Phase 2 trial tested whether inhibiting microglia with minocycline might help slow GA expansion and its corresponding vision loss.

Lonigutamab is a subcutaneously (SC) delivered humanized IgG1 monoclonal antibody targeting the insulin-like growth factor-1 receptor (IGF-1R).
Office-based surgery could prove to be a viable option for physicians.
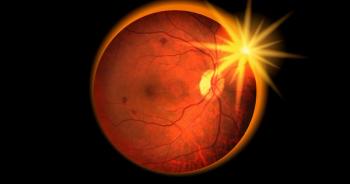
Solar retinopathy is caused by prolonged or high intensity exposure of the fovea centralis to light energy. Suspected cases of solar retinopathy may require urgent referral to an ophthalmologist for diagnosis, and to eliminate treatable causes for central visual disturbance.


In court documents in Connecticut Superior Court in Bridgeport, Isidore Sobkowski claims surgeons at the Wilton Surgery Center failed to properly clean their instruments resulting in him contracting a sight-threatening eye infection.
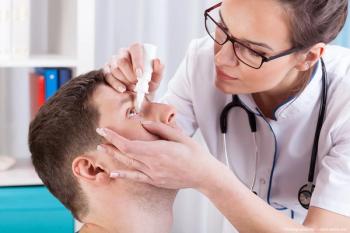
According to the company, NCX 470 is a novel NO-donating bimatoprost eye drop, is currently in Phase 3 clinical development for the lowering of IOP in patients with open-angle glaucoma or ocular hypertension.

Electrophysiologic testing combined with structural imaging to zero in on issues.

NR1D1 and NR2E3 orphan nuclear receptors were found to have low levels of expression in patients with advanced retinoblastoma.

The company noted that individuals can purchase the ARx AI headset and register for access to the Seeing AI app through the provided links or download the latest version of the NaviLens app.

The company’s EVO family of Implantable Collamer Lenses for myopia, astigmatism and presbyopia, are designed to correct vision through a minimally invasive procedure.