
The drug is the world’s first-ever CRISPR/Cas13 RNA-editing therapy for clinical use in treating neovascular age-related macular degeneration (nAMD).

The drug is the world’s first-ever CRISPR/Cas13 RNA-editing therapy for clinical use in treating neovascular age-related macular degeneration (nAMD).

Harrow announced that starting in January 2025, Vevye will be covered under key Medicare Part D formularies and across major insurance programs, expanding access to the dry eye disease treatment for millions of patients, particularly older adults.

Analysis highlights discrepancies between adult, pediatric cataract procedures.
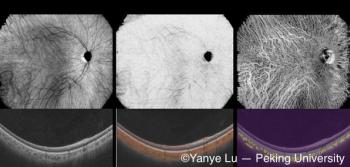
Peking University researchers have developed a deep learning-based, noninvasive choroidal angiography method that enables detailed 3D visualization of choroidal vessels from OCT scans. This technique could improve diagnostics for retinal diseases like macular degeneration, offering a safer alternative to traditional methods.

This Week in Ophthalmology is a weekly video series highlighting some of the top articles featured on the Ophthalmology Times website.
A Johns Hopkins study reveals that anti-VEGF treatments for wet AMD may unintentionally elevate ANGPTL4, a protein that promotes blood vessel growth. Combining anti-VEGF with an experimental drug targeting HIF-1 could enhance vision outcomes and prevent vision loss.

Omar Salamanca, MD, staff ophthalmologist, Orbis Flying Eye Hospital, discusses Orbis's tailored glaucoma training programs, which focus on hands-on surgical skills, early detection, telemedicine, and personalized follow-up care, helping underserved regions improve glaucoma management and blindness prevention.

Scientists at Scripps Research and its nonprofit drug development arm, the Calibr-Skaggs Institute for Innovative Medicines, are working to advance regenerative medicines capable of repairing tissues damaged by age-related disease

The phase 3 trial was conducted by licensee, Grand Pharma in Chinese cataract surgery patients.

FELIQS plans to conduct a Phase 1b/2 study of FLQ-101 (tROPhy-1 study) both in the US and Japan in the first quarter of 2025.

The Valeda Light Delivery System is for the treatment of patients with dry age-related macular degeneration (AMD).

The investigators conducted a randomized controlled trial of 60 patients who underwent monitored anesthesia care sedation for oculoplastic procedures from August 2018 to November 2020.

In an interview with David Hutton of Ophthalmology Times, Ash Abbey, MD, discusses the Phase 3 LUGANO trial for EYP-1901 in treating wet age-related macular degeneration. Abbey discusses the trial’s non-inferiority objectives, treatment schedule, patient outcomes like reduced treatment burden, and anatomical stability. Patient interest is high, particularly among those wanting fewer injections, though recruiting treatment-naive patients may be challenging. If results are favorable, potential FDA approval could occur within 3 to 4 years.

At the Tear Film and Ocular Surface meeting in Venice, Melissa Toyos shares findings on the mechanism of action of brimonidine tartrate.
To educate the public—including parents, caregivers, and allied health professionals—Prevent Blindness is offering free educational resources, including fact sheets, social media graphics, web pages, and educational videos in both English and Spanish.

New research sheds light on how the dry eye medication lifitegrast works beyond the ocular surface, targeting the underlying immune system to provide relief for patients.
Observations show distinct variations between the changes in optic nerve head (ONH) and the macula.

200 mg of caffeine elevated the blood pressure after 2 hours but did not impact the retinal VD.
A group of German researchers has discovered how tiny eye movements and the density of photoreceptors assist in visual acuity.

AMN, a rare disease, is characterized by dark reddish, wedge-shaped macular lesions

Alfredo Sadun, MD, PhD, is the chief of Ophthalmology at the Doheny Eye Institute of UCLA. At this year's AAO meeting held in Chicago, Illinois, he stopped by the Eye Care Network booth to share insights from the symposium "Professional Longevity 2.0: Assessing the Ophthalmologist," which he presented during.

This Week in Ophthalmology is a video series highlighting some of the top articles featured on the Ophthalmology Times website.

Valerie Biousse, MD, from the Emory University in Atlanta, Georgia, shared insights on how ocular imaging in the emergency department can provide timely, accurate diagnosis while also benefiting the on-call ophthalmologists. She shared these thoughts at this year's AAO meeting held in Chicago, Illinois.

In this final episode, DMEI’s residents share their future plans for expanding the device capabilities and offer practical advice to other up-and-coming creators in eye care technology.
Joanne F. Shen, MD, director of the dry eye clinic at Mayo Clinic's campus in Phoenix, Arizona, and a research team studied 35 patients treated with IPL/MGX. The team reviewed demographics, ocular histories, Standard Patient Evaluation of Eye Dryness 2 symptom survey scores, slit-lamp examinations and meibomian gland evaluations at baseline and at each visit before IPL/MGX treatments.

Researchers receive $6.4 million grant from NEI to seek new treatment for blindness-causing diseases.

Removing drops from procedure increases adherence and comfort for most patients.

NEI Initiative aims to boost awareness, standardize diagnosis, and improve care for children with CVI through multidisciplinary collaboration and emerging technologies.

The integration of artificial intelligence in laser cataract technology is shifting the paradigm for the future of cataract surgery and is driving increased precision and improved outcomes for patients.
The authors reported significant variability in DED-related symptoms, with higher prevalence rates among university students and administrative workers.