
News


Carl D. Regillo, MD, shares insights on the VERONA trial of EYP-1901 at the Retina World Congress 2025 meeting held in Fort Lauderdale, Florida.

Discover how dropless cataract surgery enhances patient comfort and outcomes, reducing the need for eye drops and improving recovery efficiency.

At Retina World Congress 2025, Dilsher S. Dhoot, MD, shares updates on the HELIOS trial and the potential future of tyrosine kinase inhibitors (TKIs)

Researchers uncover corneal changes in multiple myeloma patients treated with belantamab mafodotin, revealing potential impacts on vision and ocular health.

RWC 2025: Katherine Talcott, MD, discusses the potential connection of auto-immune conditions to AMD
This large database study explored the link between autoimmune problems and AMD.

In this conversation, Franklin discussed a research study on intraoperative fluorescein angiography in surgical procedures, highlighting significant advancements in surgical visualization and patient outcomes.

Glaucoma management is shifting toward earlier, drop-sparing interventions using sustained drug delivery and advanced laser technologies that improve outcomes and quality of life for both patients and physicians.
Optigo Biotherapeutics presents promising preclinical data at ARVO.

Innovent Biologics initiates phase 2 trial for efdamrofusp alfa, a promising treatment for diabetic macular edema, aiming to improve patient outcomes.

Machine learning algorithms enhance retinopathy of prematurity screening using smartphone images, expanding access in low-resource settings and easing pediatric ophthalmologist workloads.

The VAN-2401 phase 1 clinical trial will evaluate the use of KH658 for the treatment of wet AMD.

Galimedix Therapeutics advances GAL-101, an oral therapy targeting Alzheimer’s and AMD, completing phase 1 SAD study with promising safety results.

Exploring how oral sedation and office-based surgery can enhance efficiency and patient experience

A one-drop, low dose atropine study assesses accommodative amplitude and dynamics, with clinically applicable findings.


Topcon Healthcare launches IDHea, an innovative platform enhancing AI research and ocular data access to improve clinical outcomes in healthcare.
A study reveals that repeated anti-VEGF injections for AMD do not alter retinal vascular metrics over time, ensuring treatment stability.
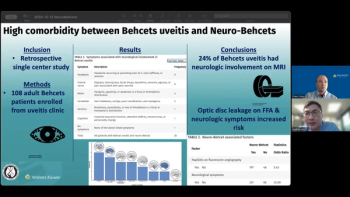
In cases with optic nerve hyperfluorescence or neurologic symptoms, prompt neuroimaging is warranted to rule out neuro-Behcet disease and guide multidisciplinary management.

Katherine Talcott, MD, a retina specialist at Cleveland Clinic, presented findings on EYP-1901 in the phase 2 DAVIO study.

The trial will assess allogeneic retinal pigment epithelium cells in the treatment of geographic atrophy.

In this Q&A, Luke Lindsell, OD, MD, shares his insights on emerging therapies in retina, the evolving role of optometrists in retinal disease management, and the importance of comanagement in patient care.

The first SightLine meeting, held pre-ASCRS, reveals insights on physician-industry relationships, mentorship opportunities, and the practical applications of AI in healthcare.

Opus Genetics reveals promising 1-year results from OPGx-LCA5 gene therapy, showing sustained vision improvements in adults with LCA5 retinal degeneration.

Outcomes from high myopia LASIK using the Teneo laser platform demonstrate strong visual stability and patient satisfaction, with reduced dysphotopsias and minimal reliance on cycloplegia or nomograms.
New research reveals subretinal drusenoid deposits as potential indicators of serious heart disease, highlighting the need for ophthalmologists to screen patients effectively.



