
The study will be led by principal investigator professor Noemi Lois, MD, PhD, FRCS(Ed), FRCOphth, at Queen’s University Belfast, in over 20 clinical sites, and plans to enroll 264 patients across the UK with severe DME.

The study will be led by principal investigator professor Noemi Lois, MD, PhD, FRCS(Ed), FRCOphth, at Queen’s University Belfast, in over 20 clinical sites, and plans to enroll 264 patients across the UK with severe DME.

Through the program, gene therapies are developed to treat patients with retinitis pigmentosa caused by pathogenic variants in the MERTK gene.

From Nobel laureates to AI-driven research, the Wilmer Eye Institute honors a century of transforming vision science and care.
The trial is evaluating KB803 for the treatment and prevention of corneal abrasions in dystrophic epidermolysis bullosa

ADX-2191 is an investigational drug candidate from Aldeyra, for the treatment of primary vitreoretinal lymphoma (PVRL), a rare and potentially fatal cancer currently with no current FDA-approved therapy

New findings reveal RG6501 cell therapy shows promising long-term visual improvements for geographic atrophy patients
Phentolamine Ophthalmic Solution 0.75% is a non-selective alpha-1 and alpha-2 adrenergic antagonist to reduce pupil size, administered as an eye drop for the treatment of presbyopia.

FYB203 has been approved by the US FDA and UK Medicines and Healthcare products Regulatory Agency for the treatment of neovascular age-related macular degeneration (nAMD), diabetic macular edema, macular edema following retinal vein occlusion, and visual impairment due to myopic choroidal neovascularization.
ST-100 shows promise as a fast-acting treatment for dry eye disease, offering rapid relief and unique collagen repair mechanisms.

Danegaptide is an oral therapy for early treatment of non-proliferative diabetic retinopathy (NPDR) and associated edema.
Ixo-vec shows sustained efficacy and reduces injection burden through 4 years in clinical trials
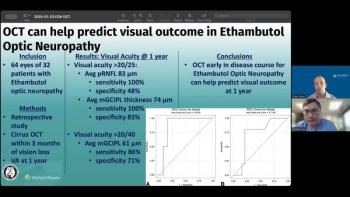
Andrew G. Lee, MD, and Drew Carey, MD, discuss how baseline optical coherence tomography parameters can help ophthalmologists counsel patients and make more informed decisions after ethambutol-associated optic neuropathy.
Intracameral tPA can treat fibrinous clots in severe anterior uveitis.
As its director, Manusis outlines the facility’s role in delivering advanced, personalized solutions to improve vision and patient experience.
Changing demographics and increasing trial complexity present challenges and opportunities.

The report divides the research into 7 categories: sex, gender, and hormones; epidemiology; pathophysiology; tear film; pain and sensation; iatrogenic; and clinical trial design.

Patients in Ontario expose illegal user fees for cataract surgeries in private clinics, urging government action to uphold the Canada Health Act.

ZinserLab will focus on accelerating the development of imaging technologies in eye care.

From laser vision correction to adjustable lens implants, the Center for Refractive Solutions at New York Eye and Ear Infirmary of Mount Sinai offers advanced options for every stage of life.

To conduct the study, the investigators obtained data on coffee consumption from genome‐wide association studies (GWAS) and the latest AMD‐related GWAS summary data from the Finngen consortium R11.

Funding will support continued product development, while the board is composed of “global leaders in posterior and anterior segment eye care, artificial intelligence and clinical trial design.”
A look at current therapies, clinical trial results, patient education, and emerging options for managing this progressive retinal disease.

Assistive devices empower patients with central vision loss to regain independence, enhancing daily activities and reducing reliance on low vision centers.

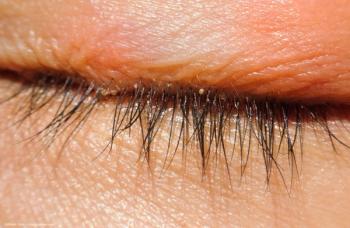
Timely diagnosis and personalized treatment are key to improving visual outcomes.

With detailed imaging and cognitive data, the Northern Ireland Cohort for the Longitudinal Study of Aging highlights the potential of integrating eye scans into broader health research.

Optical coherence tomography markers—like the ellipsoid zone—are reshaping clinical trials in intermediate age-related macular degeneration, as highlighted at the 2025 International SPECTRALIS Symposium — And Beyond (ISS).

Advanced monitoring strategies are overcoming significant obstacles in retinal care, as highlighted at the 2025 International SPECTRALIS Symposium — And Beyond (ISS).

The trial treated 9 patients in 3 sequential, ascending dose-level (DL) cohorts.