
The authors noted that the proportion of patients losing fewer than 15 letters from the baseline BCVA score in the study eye was comparable between the two groups.

The authors noted that the proportion of patients losing fewer than 15 letters from the baseline BCVA score in the study eye was comparable between the two groups.

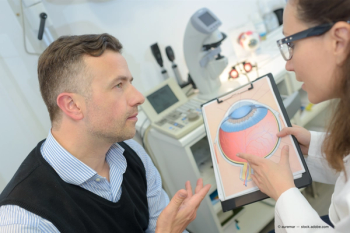
Elizabeth Yeu, MD, will highlight pearls for managing intraocular lenses in difficult cases.

The Eye Center at UC Davis Health has two new endowed chairs focused on glaucoma, thanks to a $4 million donation from business entrepreneur Daryl Geweke.

Ophthalmologists should instruct their patients to pay attention to their eye health for the long term.

In AMD research, the experimental animals used are often young male mice. This is not optimal for the development of new treatments, as the disease most often affects the elderly – and women.

Claes H. Dohlman, MD, PhD, widely considered the father of modern cornea science, is a recipient of the 2022 António Champalimaud Vision Award. The award, considered the “Nobel Prize of Vision," comes with a $1 million prize .

According to Rishi P. Singh, MD, family history and lifestyle are key in the development of geographic atrophy.

LUNA trial will evaluate the 2x10^11 vg/eye (2E11) and a new lower 6x10^10 vg/eye (6E10) dose of Ixo-vec, with enhanced prophylactic steroid regimens in patients requiring frequent anti-VEGF injections. Interim data anticipated throughout 2023.

Heru debuts dark adaptation, the newest modality added to its wearable health and wellness platform’s vision screening line-up.

Medication is administered after LASIK and injectable prophylaxis during cataract surgery.

No one should ever have to choose between quality health care and preserving money, especially for those we love or for ourselves.


Sigma extends access to powerful no-code cloudscale data analytics for industry-compliant data cloud so healthcare professionals and researchers can find faster answers to critical questions.
The inflationary economy and lasting effects of COVID-19 have caused staff and product shortages, a rise in labor costs, and increased patient sensitivity to medication costs.

The ScoutPro system allows eye care practices to minimize tech time and seamlessly integrate osmolarity testing into any practice configuration or patient protocol.

According to the company, the NDA submission for Nyxol eye drops in first indication is on track for late 2022.

Haiyan Gong, MD, PhD, professor of ophthalmology and anatomy and neurobiology at Boston University School of Medicine, has received $200,000 through a Standard Award in National Glaucoma Research from the BrightFocus Foundation.

Zeiss parlays technology into standardization for ophthalmologists and their patients
Scientist have proposed several methods for converting stem cells into RPE, but there is still a gap in our knowledge of how cells respond to these stimuli over time.
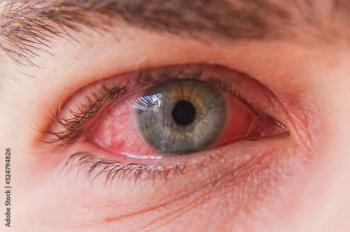
Spanish investigators from the Ophthalmology Service, Hospital Clínico San Carlos, Instituto de Investigación Sanitaria del Hospital Clínico San Carlos, Madrid, led by first author Fernando Ly-Yang, MD, reported the case of a 42-year-old man with monkeypox.

The research, presented at the 22nd EURETINA World Congress, outlined that patients with RP experience greater levels of stress and anxiety than individuals in the general population and that the level of stress can be measured easily and non-invasively in hair.

During the 22nd European Society of Retina Specialists Congress in Hamburg, Germany., investigators noted that Yusar and colleagues from the Vitreoretinal Services, Centre for Sight, Delhi, India.

Oral ZPX3330 found to demonstrate favorable safety and tolerability profile in interim masked safety results, consistent with 11 Prior Trials of APX3330.

According to the company, the Phase 3 pivotal trials met pre-specified primary efficacy endpoints for both doses of iDose TR., supported an anticipated upcoming NDA submission.

Elisabeth J. Cohen, MD, has been appointed vice chair for academic affairs, enhancing the department’s commitment to research and advancing its reputation for excellence in studying and treating diseases of the eye.

Regeneron announced that the primary endpoints were met in two pivotal trials investigating novel aflibercept 8 mg with 12- and 16-week dosing regimens in patients with diabetic macular edema and wet age-related macular degeneration.

Craig Morgan, MD, and Eye Consultants of Huntington, West Virginia, paid $907,074.64 to resolve allegations that they submitted false claims to Medicare and Medicaid, the U.S. Attorney’s Office for the Southern District of West Virginia reported.

If approved, TP-03 may offer treatment for millions of patients with Demodex blepharitis. TP-03 is now also being studied for the treatment of Meibomian Gland Disease in patients with Demodex mites.
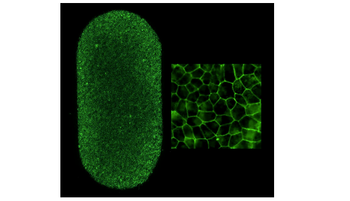
According to the National Institutes of Health, the therapy was derived from the patient’s blood by converting blood cells to iPS cells which were then programmed to become retinal pigment epithelial cells, which were surgically implanted as a patch of tissue.
GATHER2 met its prespecified primary endpoint of mean rate of growth (slope) in GA area at 12 months with statistical significance and a favorable safety profile. With this data in hand, Iveric Bio is working to submit a New Drug Application for Zimura to the FDA by the end of the first quarter of next year.