
The researchers from Indiana University School of Medicine found neurons use mitochondria for a steady source of energy, and restoring mitochondrial homeostasis in the diseased neurons can protect the optic nerve cells from being damaged.

The researchers from Indiana University School of Medicine found neurons use mitochondria for a steady source of energy, and restoring mitochondrial homeostasis in the diseased neurons can protect the optic nerve cells from being damaged.
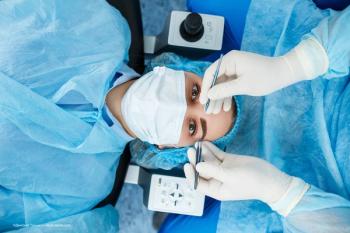
ICLs offer a strong surgical correction option for some patients.

Horizon Therapeutics presented data at the North American Neuro-Ophthalmology Society (NANOS) Annual Meeting, being held through March 16 in Orlando, Florida, summarizing real-world experience of people living with TED and DON who were treated with teprotumumab-trbw between January 2020 and September 2022.

While low-dose atropine eye drops are currently being used to slow myopia progression in several countries in Asia, researchers set out to determine if the medication could also the onset of myopia.

The researchers focused on photoreceptor cells (PRCs), which are light-detecting cells found in the retina.

A look at what’s in the therapeutic delivery pipeline for these disorders.

According to the U.S. District Court, during the jury trial before U.S. District Judge Wilhelmina M. Wright, the United States proved that the defendants the Cameron-Ehlen Group, Inc., which does business as Precision Lens, and its owner Paul Ehlen paid kickbacks to ophthalmic surgeons to induce their use of Defendants’ products in cataract surgeries reimbursed by Medicare.

Celebrating 40-year anniversary with new brand/new products.
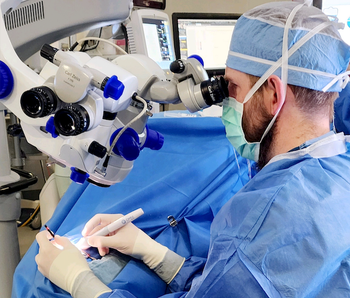
The Ergo-Series of the OMNI Surgical System maximizes ergonomics and surgical ease-of-use while continuing to enable surgeons to perform minimally invasive, implant-free glaucoma procedures.

A team of researchers compared 10-MHz and 15-MHz B-scan probes to evaluate the probes’ ability to detect and localize retinal detachments in eyes filled with silicone.

According to the company, the funding supports the Phase 2 clinical study of ONL1204 ophthalmic solution to evaluate the safety and efficacy in patients diagnosed with macula-off rhegmatogenous retinal detachment.

Avenova Eye Health Support oral supplement aims to comfort dry eyes and support overall eye health.

The 2023 Harrington Prize for Innovation in Medicine recognizes Jean Bennett, MD, PhD, and Albert M. Maguire, MD, for their work in retinal gene therapy for genetic diseases.
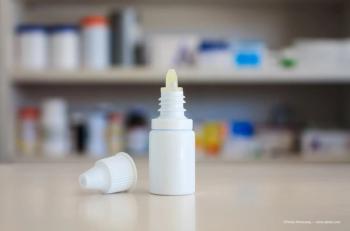
The company’s Purely Soothing eye drop is used as an anti-inflammatory used to assist with symptoms of ocular irritation and/or swelling, including dry eye.

The month will help to bring awareness to eye safety in the workplace and keep eyes safe and protected on the job.
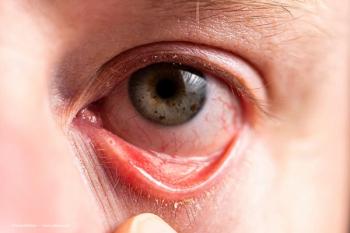
Alice Epitropoulos, MD, gives some insight into meibomian gland dysfunction (MGD), one of the most prevalent forms of dry eye.
The voluntary recall is being issued out of an “abundance of caution” due to cracks that have developed in some of the units caps of the bottles.
Under the terms of the agreement, Grifols to have worldwide exclusive commercial rights to Selagine’s treatment upon regulatory authorization, expected in early 2029.

Alice Epitropoulos, MD, gives tips on how to optimize treatment for patients with meibomian gland dysfunction (MGD).
Researchers have identified a new target to prevent the formation of abnormal tangles of blood vessels associated with eye conditions such as neovascular age-related macular degeneration, proliferative diabetic retinopathy and ischemic retinal vein occlusion.

Researchers recommended that patients being treated with ethambutol should be screened regularly by an ophthalmologist for the early detection of optic neuropathy.

According to researchers, the treatment with botulinum toxin is safe, fast-acting, and improves both cosmetic appearance and quality of life, and could be considered as a choice for patients not opting for surgery.

Researchers conducted a retrospective analysis of longitudinal, clinical data from patients with high myopic MNV treated with intravitreal bevacizumab.

The event will bring together patients, care partners, allied personnel, and medical professionals to speak directly with their elected officials to discuss a variety of vision and eye health issues

Ophthalmologists could find themselves treating a growing number of young people who are applying lip balm to their eyelids, only to experience discomfort and vision issues when it gets into their eyes.

According to the company, post-hoc time-to-event analysis signals up to 59% risk reduction in rate of vision loss compared to sham treatment at 12 months. The analysis will be presented at the ARVO Annual Meeting from April 23-27 in New Orleans.
According to researchers, patients with chronic CSC treated with 1 fovea-involving, half-dose PTD application had a significant improvement in structure and function at the final follow-up and foveal atrophy did not develop in any patients.

Key is to use stem cell treatments to ensure optimal outcomes for patients.

According to James Chodosh MD, MPH, some corneal conditions may not be treatable with a transplant.
At the American Glaucoma Society meeting in Austin, Texas, the company presented findings demonstrating the safety and efficacy of its FLigHT treatment.