
Martin David Harp

Editor, Ophthalmology Times
Articles by Martin David Harp


LENZ Therapeutics previously licensed Greater China rights to CORXEL for the development and commercialization of LNZ100 in April 2022.

To mark Ophthalmology Times' 50th anniversary, we invited top experts to reflect on the most significant innovations in ophthalmology over the past five decades.

To mark Ophthalmology Times' 50th anniversary, we invited top experts to reflect on the most significant innovations in ophthalmology over the past five decades.


Vonaprument has received fast track designation from the US Food and Drug Administration.

To mark Ophthalmology Times' 50th anniversary, we invited top experts to reflect on the most significant innovations in ophthalmology over the past five decades.
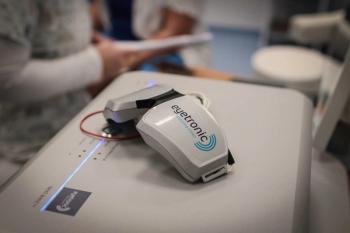
Eyetronic is a noninvasive treatment for glaucoma that provides external neural stimulation to the optic nerve of patients.
OpenAI's ChatGPT-4o enhances ophthalmological image generation, producing realistic retinal photographs while highlighting the need for further research in training datasets.

To mark Ophthalmology Times' 50th anniversary, we invited top experts to reflect on the most significant innovations in ophthalmology over the past five decades.

Viatris' phase 3 trial of pimecrolimus 0.3% (MR-139) for blepharitis fails to meet primary end point
The primary end point of the trial was complete resolution of debris after 6 weeks of twice-daily dosing.

Funding will be specifically used to accelerate the clinical development of urcosimod (formerly called OK-101).
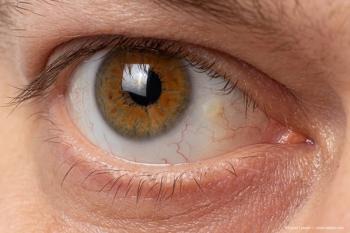
Pinguecula is a benign, common degeneration of the conjunctiva that appears as a grey-white-yellow mass on the bulbar conjunctiva.

Under the terms of the agreement, Kowa receives exclusive rights to develop and commercialize NCX 470 in the US and all other territories of the world excluding Japan, China, Korea, and Southeast Asia.

Loteprednol etabonate ophthalmic suspension, 0.5% is bioequivalent to lotemax ophthalmic suspension, 0.5%, of Bausch & Lomb.

To mark Ophthalmology Times' 50th anniversary, we invited top experts to reflect on the most significant innovations in ophthalmology over the past five decades.

Urcosimod is a lipid conjugated chemerin peptide agonist of the ChemR23 G-protein coupled receptor.

Included in the agreement are ranibizumab-nuna 0.05 mL injection (BYOOVIZ), referencing LUCENTIS (ranibizumab), and aflibercept-yszy 0.05 mL injection (OPUVIZ), referencing EYLEA (aflibercept).

A Prescription Drug User Fee Act (PDUFA) target action date of December 16, 2025, was assigned by the FDA.

To mark Ophthalmology Times' 50th anniversary, we invited top experts to reflect on the most significant innovations in ophthalmology over the past five decades.
Krystal Biotech initiates EMERALD-1 trial for KB801, a redosable eye drop gene therapy.

To mark Ophthalmology Times' 50th anniversary, we invited top experts to reflect on the most significant innovations in ophthalmology over the past five decades.

The VISTA trial is evaluating and comparing 2 dose levels of laru-zova with an untreated control group for the treatment of X-linked retinitis pigmentosa (XLRP).


The company notes that the workforce reduction is expected to provide annual cash compensation cost savings of approximately $15 million and offsets additional expenses expected based on the accelerated timelines for the 4FRONT clinical trials.

LumiThera’s PBM is the only device that has demonstrated meaningful vision improvement compared to baseline for people living with early to intermediate dry AMD.

AAV204 is a novel adeno-associated virus (AAV) capsid from the AIM capsid library licensed by Abeona from the University of North Carolina at Chapel Hill.

The program allows patients to undergo genetic testing and high-resolution retinal imaging anonymously and provide insights into both ocular and systemic diseases.
Yesafili is the first biosimilar to Eylea to be approved by Health Canada.

RGN-259 is a thymosin beta-4–based eye drop designed to rival Dompé’s Oxervate, the only FDA-approved prescription eye drop to treat people with neurotrophic keratitis.

