
STAAR Surgical extends its merger timeline with Alcon, inviting third-party proposals to maximize stockholder value amid ongoing opposition from Broadwood Partners.

Editor, Ophthalmology Times

STAAR Surgical extends its merger timeline with Alcon, inviting third-party proposals to maximize stockholder value amid ongoing opposition from Broadwood Partners.

This resubmission follows the complete response letter (CRL) Outlook received in August 2025.



The BIM-IOL System is for the treatment of elevated IOP in patients with mild-to-moderate open-angle glaucoma (OAG) or ocular hypertension (OHT).
The company describes KSI-101 as a “novel, potent, and high-strength (100 mg/mL) antibody-based investigational therapy with a bispecific mechanism of action targeting both interleukin-6 (IL-6) and vascular endothelial growth factor (VEGF).”

OPGx-LCA5 is designed to address a form of Leber congenital amaurosis that results from biallelic mutations in the LCA5 gene, which encodes the lebercilin protein, the investigators explained.

PolyActiva and RareSight collaborate to create innovative therapies for rare pediatric retinal diseases, aiming to transform treatment and improve children's vision.

The trial is evaluating 4D-150 in patients with wet age-related macular degeneration (wet AMD).

The VITA Trial is a prospective pilot study on the VisiPlate Aqueous Shunt in patients with with open-angle glaucoma.

The EXTEND study is a 5-year follow-up of participants who received a single intravitreal injection of MCO-010 in a previously conducted phase 1/2a trial.

The trial will be evaluating AXPAXLI dosed every 6 months versus aflibercept (2 mg) dosed every 8 weeks in treatment-naïve wet AMD patients.

Under the terms of the agreement, 4DMT will grant Otsuka exclusive rights to develop and commercialize 4D-150 for retinal vascular diseases in Japan, China, Australia, and other Asia-Pacific markets.

The funding will support the completion of the clinical PoC of its AAVB-039 CELESTE study and the completion of the STELLA natural history study

Veligrotug is an intravenously delivered, anti-insulin-like growth factor-1 receptor (IGF-1R) antibody for the treatment of thyroid eye disease (TED).
To celebrate Ophthalmology Times' 50th anniversary, we asked leading experts what the practice would look like today had optical coherence tomography (OCT), one of the biggest innovations in the field, never been invented.

To celebrate Ophthalmology Times' 50th anniversary, we asked leading experts what the practice would look like today had optical coherence tomography (OCT), one of the biggest innovations in the field, never been invented.
NYRVANA is the first-in-human trial and is an open-label, multicenter, dose-escalation study investigating the safety, tolerability, and preliminary efficacy of a single intravitreal injection of SPVN20 over 6 months.

To celebrate Ophthalmology Times' 50th anniversary, we asked leading experts what the practice would look like today had optical coherence tomography (OCT), one of the biggest innovations in the field, never been invented.

To celebrate Ophthalmology Times' 50th anniversary, we asked leading experts what the practice would look like today had optical coherence tomography (OCT), one of the biggest innovations in the field, never been invented.
STAAR releases new date for shareholder meeting

Xelafaslatide (formerly ONL1204) is a small-molecule Fas inhibitor designed to protect key retinal cells from cell death that occurs across multiple retinal diseases and conditions such as geographic atrophy.
To celebrate Ophthalmology Times' 50th anniversary, we asked leading experts what the practice would look like today had optical coherence tomography (OCT), one of the biggest innovations in the field, never been invented.
REMAIN is the long-term extension of the phase 2b/3 RESTORE trial evaluating MCO-010 in retinitis pigmentosa (RP).

The CNPV program was announced by the FDA in June 2025 and offers companies the opportunity to reduce standard application review times from 10–12 months to just 1–2 months.

According to the company, FALCON aimed to provide “a better understanding of how autosomal dominant optic atrophy (ADOA) disease parameters change over time to inform potential future interventional clinical trials.”

The trial evaluated the safety, efficacy, and tolerability of AURN001 in patients with corneal edema secondary to corneal endothelial dysfunction.

Broadwood stresses that STAAR ignored offers outside Alcon, while STAAR states Broadwood Partners’ claims are "misleading and distort the truth."

DURAVYU is being developed as a potential sustained-delivery treatment for patients suffering from serious retinal diseases.
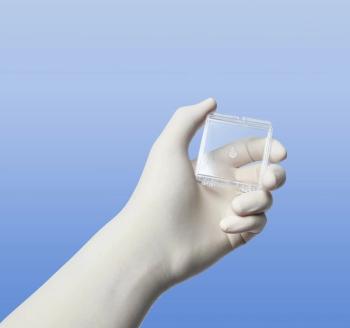
The VisiPlate aqueous shunt is made of a patented metamaterial that has been designed to be non-fibrotic and is many times thinner than a human hair, according to the company.