Oculis reported positive data from two of its clinical proof-of-concept phase 2 trials for OCS-02, a novel topical anti-TNF alpha antibody fragment candidate, for the treatment of dry eye disease and acute anterior uveitis.

Oculis reported positive data from two of its clinical proof-of-concept phase 2 trials for OCS-02, a novel topical anti-TNF alpha antibody fragment candidate, for the treatment of dry eye disease and acute anterior uveitis.

Johnson & Johnson Vision announced today that the FDA has granted 510(k) clearance and CE mark for its VERTIAST Vision System, a next-generation phacoemulsification innovation.

Find out how one eye care practice has found success in adapting their services to ensure staff/patient safety throughout the pandemic.
Bausch + Lomb and Novaliq GmbH have announced statistically significant topline data from the first phase 3 trial analyzing the investigational drug NOV03 as a first-in-class eye drop to treat dry eye disease associated with MGD.

A diminishment of both hearing and vision is linked to an increased risk for dementia and mental decline, according to a new study.

Mary Elizabeth Hartnett, MD, FACS, FARVO, and Mark Breazzano, MD, discuss current research being conducted involving AMD and maximizing patient outcomes amid the pandemic.

Drinking alcohol on a regular basis may decrease patients’ chances of developing cataracts that require surgery, new research shows.

Clinical-stage pharma company announces next step in investigation of BRIMOCHOL to treat presbyopia.
Ophthalmologists share the most pressing issues on their mind within the glaucoma space — and why losing sleep from time to time may actually be a good thing.
Visus Therapeutics announced today that FDA has approved its Investigational New Drug Application (IND) to move forward with the clinical development program for BRIMOCHOL

Graybug Vision, Inc. has reported preliminary topline data from a 12-month treatment phase of its Phase 2b ALTISSIMO trial of GB-102 for the treatment of wet AMD.

In this latest EyePod® episode, Sahar Bedrood, MD, PhD, shares her clinical perspective on the multi-faceted, dynamic evolution of interventional glaucoma.

The FDA has accepted Oyster Point Pharma's New Drug Application (NDA) for its OC-01 (varenicline) nasal spray to treat dry eye disease.
Findings from a new quality of life (QOL) assessment on thyroid eye disease (TED) indicate that the impact on patients extends well beyond its active phase and into the chronic phase.

In this latest EyePod episode, John H. Merey, MD, possibly one of the last Holocaust survivors still in the practice of ophthalmology, shares his experience.
Biophytis will begin recruiting patients in France and Belgium for the second part of the COVA Study analyzing its leading drug candidate as a potential treatment for acute respiratory failure linked to COVID-19.
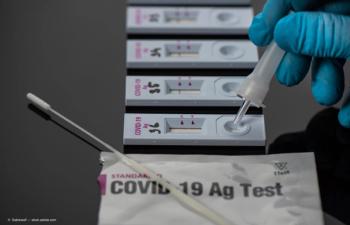
Rapid test allows healthcare professionals to make fast decisions at the point of care.
Glaukos Corporation's COO Chris Calcaterra gives an update on the company's latest 24-month phase 2b data for its iDose TR sustained-release travoprost implant, next steps for securing FDA approval, and his outlook for 2021.

Donald L. Budenz, MD, MPH, shares the key takeaways from his Shaffer-Hetherington-Hoskins Lecture, focusing on glaucoma epidemiology, presented during the 2021 Glaucoma 360's 25th Annual Glaucoma Symposium CME.

The FDA has granted Fast Track designation to MeiraGTx's AAV-CNGA3 gene therapy product candidate for the treatment of achromatopsia (ACHM).

Ocular symptoms in COVID-19 patients may be more common than previously thought — with sore eyes a significant sign of disease.

Robert L. Stamper, MD, discusses the findings of a study analyzing the effects of corneal mechanics on IOP, presented during the virtual Glaucoma 360 meeting.

During the 2021 Glaucoma 360 meeting, Ruth D. Williams, MD, speaks on the highlights and key findings from the EAGLE study, which showed clear lens extraction surgery may be a better initial treatment for some glaucoma patients.

Neurophth Therapeutics, Inc (Neurophth) and AAVnerGene Inc have announced the launch of a strategic partnership that will grant Neurophth global rights to mutually select adeno-associated virus (AAV) capsids for the creation of the next-generation ophthalmic gene therapy.

See what ranked most popular in our top 10 stories of 2020.
From patient care and treating glaucoma to the ongoing pandemic, ophthalmologists share the most pressing thoughts of concern leaving them awake at night.

Amydis announced the successful completion of a pre-investigational new drug (IND) meeting with the FDA for its candidate AMDX-2011P, a small-molecule retinal tracer that targets amyloid beta to diagnosis amyloid angiopathy.

Two teams of researchers from Gyroscope Therapeutics and the University of Pennsylvania are joining forces to explore gene therapy targets for three specific serious eye diseases.
Topline data following completion of ALTISSIMO trial treatment phase expected in second quarter of 2021.