
Jeff Todd talks about the campaign and partnering with actress Debbie Allen to help gain awareness on eye health.

Jeff Todd talks about the campaign and partnering with actress Debbie Allen to help gain awareness on eye health.

The system is a next generation aqueous shunt therapy for patients with moderate to severe glaucoma.

According to the company, 4D-150 comprises its customized and evolved intravitreal vector, R100, and a transgene cassette that expresses both aflibercept and a VEGF-C inhibitory RNAi.
According to the company, Steven Fusco will focus on Bruder Healthcare and Hilco Vision product portfolios.

AnnMarie Hipsley, DPT, PhD, sat down to speak about founding iAware, a non-for-profit organization established for the promotion of awareness of age-related ocular disease.

According to the company, the deal expands its European footprint into Spain and Portugal.

The beauty brand announces Ashley Brissette, MD, MSc, FRCSC, will be the brand's first guiding ophthalmologist.

According to the company, NFS-05, utilizes a gene therapy approach that delivers an AAV vector containing the OPA1 gene into the vitreous cavity.
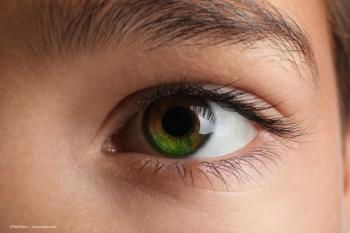
The 6-month-old boy’s dark brown eyes turned deep blue after he was treated for COVID with favipiravir.

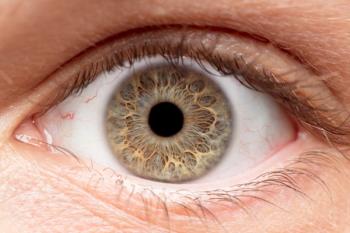
Brimonidine is an alpha-adrenergic receptor agonist indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.

According to the company, the tool provides free access to training and mentors for eye care professionals in areas with the greatest need, and the new Cybersight mobile app expands access for eye care professionals through offline functionality.

Physician has blazed career path in ophthalmology, breaking glass ceiling.

The estate of James Sams, filed a lawsuit against Lowrey King, MD, Roper St Francis Healthcare, The Retina Eye Center of Charleston, and Carolina Eye Care Physicians in a South Carolina court.
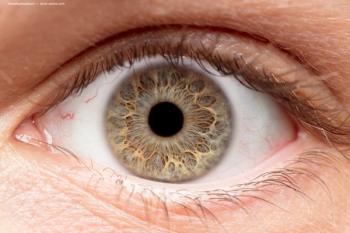
Study demonstrates power of IRIS Registry for evaluation of safety, efficacy

Laura Kopplin, MD, PhD, spoke with our team to share insights from her presentation at the Women in Ophthalmology Summer Symposium 2023 in Marco Island, Florida.

Take a look at a review of the highlights and hottest stories from Ophthalmology Times during the week of August 27, 2023.
The study of pathological tissues will enable to establish the role of corneal structure in certain diseases.

"What research at the 2023 ASRS meeting do you find exciting or interesting?" Here's what Aaron Lee, MD, Megan Baldwin PhD, and Carl Danzig, MD had to say.


Prevent Blindness plans to educate the public on the risk of significant eye injuries and the need for the proper sports eye protection, with more than 32,000 sports-related eye injuries treated in 2022, up almost 20 percent from previous year.
Researchers find gray GA lesions had the highest cross-modality differences

According to the university, a special antibody that is derived from llamas, called a nanobody, can stop the misfolding and the activation of Rhodopsin, a molecule whose mutations can lead to blindness.

According to the company, full enrollment anticipated by first week of September. The phase 2 trial is designed as potential registration trial with pre-specified primary efficacy endpoints covering both a sign and symptom of DED.

We asked, "What research at ASRS 2023 do you find exciting or interesting?" Here's what Paul Hahn, MD, PhD, Kerrie Brady, BPharm, MBA, MS, and Michael Singer, MD had to say!

The trial will be conducted by French tissue bank, TBF Génie Tissulaire.
According to the Keck School of Medicine of USC, the $12.4 million from the California Institute for Regenerative Medicine is the latest round of support for USC researcher Mark Humayun and a milestone in the development of a stem cell patch to treat advanced dry age-related macular degeneration.

In this podcast episode, Ophthalmology Times' co-chief medical editors Peter J. McDonnell, MD, and Neda Shamie, MD, delve into the clinical nuances of Demodex blepharitis. Through personal anecdotes, they stress its underrecognized gravity and discuss diagnostic cues like collarettes. The experts share their enthusiasm for improved treatments, echoing the transformative impact seen in dry eye care.

According to Outlook Therapeutics, it is working with the FDA to address the agency’s issues.

The study from New Zealand was conducted on an otherwise healthy 28-year-old woman.