
The MyopiaX-1 trial studies MyopiaX, a smartphone application that aims to slow the progression of myopia, or nearsightedness, in children and adolescents.

The MyopiaX-1 trial studies MyopiaX, a smartphone application that aims to slow the progression of myopia, or nearsightedness, in children and adolescents.

Experts in the field weigh in on and give opinions on the question "What do you wish patients knew about eye health."
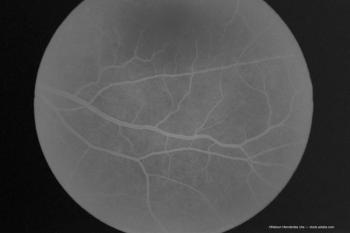
Investigators note a supply shortage led to use of half a dose for imaging purposes.

In this EyePod® episode, David Hutton sits down with Shankar Musunuri, CEO of Ocugen to discuss updated data for OCU400.

The organization is warning the public about the hazards of misusing contact lenses. Contact lens wearers also must take extra care when applying and removing eye cosmetics.

The effort will capture the real-life stories of patients and eye care professionals around the world.

According to the company, the divestment includes Xiidra, on-market treatment for dry eye disease and additional ophthalmic investigational therapies. Novartis will now move forward with a focused portfolio and prioritized therapeutic areas for future growth.

A team of University of Rochester scientists develop computer models of patients’ eyes to identify the ideal IOLs and visual simulators for patients to experience how they will see with them.


According to Outlook, in the FDA’s recently issued CRL it concluded it could not approve the BLA during this review cycle due to several CMC issues, open observations from pre-approval manufacturing inspections, and a lack of substantial evidence.
The specialty pharmaceutical research company’s new retinal vascular dysfunction model can have an impact on the research and development of new drugs for retinal vascular diseases.

In cases where patients struggle with applying or tolerating eye drops, especially after previous selective laser trabeculoplasty (SLT) treatment, MicroPulse transscleral laser therapy (TLT) offers a practical solution.

Kriya is developing a one-time gene therapy designed to block complement C3 and C5, which are clinically validated substrates targeted by FDA approved therapies, to delay the progression of geographic atrophy.

Efficacy of treatment option is also confirmed in pediatric population in India.
The Lasker-DeBakey Clinical Medical Research Award, dubbed ‘America’s Nobel,’ recognizes the wide use of optical coherence tomography to manage eye disease, prevent blindness.

The company announced its previously announced agreement to divest its late-stage ophthalmic assets to Laboratoires Théa S.A.S. has been terminated.

Ocuphire Pharma and Viatris developed the drug together for the reversal of pharmacologically-induced mydriasis (RM) produced by adrenergic agonist or parasympatholytic agents.
Preservative-free Iyuzeh, launched more than 10 years ago, currently is available in 46 countries, mostly under the brand name Monoprost, with about 1.5 million patients treated monthly, according to the company.

Discover how advocacy can impact your clinical work and ultimately patient care.
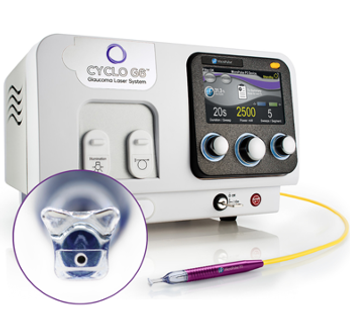
Highlighting the benefits of Iridex's updated P3 Probe and Cyclo G6® Laser software for MicroPulse® transscleral laser therapy. The redesigned probe improves ergonomics and visibility, while the user-friendly software ensures precise, reproducible glaucoma treatments.

Study finds 45% of injuries involve the eyes.

Peter J. McDonnell, MD, and Neda Shamie, MD, discuss the transformative impact of minimally invasive glaucoma surgery (MIGS) on glaucoma care and its integration with cataract surgery, sharing insights and practical tips for ophthalmologists.

Procedure corrects small-angle strabismus and removes need for prism glasses

Sometimes, the best job for you is the one you create.

On the 'Negotiations' episode of RWO: The Podcast, Dr. Lisa Nijm joins Drs. Nicole Bajic and Grayson Armstrong to discuss personal experiences with contract negotiation.
According to a news release, the J-code for SYFOVRE will become effective on October 1, 2023.
The Orbis Flying Eye Hospital will return to Zambia for a 3-week training project in Lusaka coinciding with World Sight Day on October 12.
According to the companies, the partnership will roll out a proprietary system that has earned FDA breakthrough designation, one of the few ophthalmic devices to achieve this status in almost a decade.


To educate patients on the disease, the company has enlisted the help of actor Eric Stonestreet and his mother, Jamey.