Repeated 0.1-ml intravireal injections of lampalizumab, antigen-binding fragment of a humanized monoclonal antibody that inhibits complement factor D, seem to be associated with a low risk of elevated IOP

Repeated 0.1-ml intravireal injections of lampalizumab, antigen-binding fragment of a humanized monoclonal antibody that inhibits complement factor D, seem to be associated with a low risk of elevated IOP

The biosimilar was approved for treating patients with age-related neovascular (wet) macular degeneration (nAMD) and other serious retinal diseases.

Plasma emission for blepharitis, meibomian gland dysfunction, and dry eye disease.

Ashley Brissette, MD, MSc, FRCSC, team ophthalmologist for the New York Rangers sat down to talk about eye injuries in hockey and other professional sports, as well as advancements in prevention and treatment for the players.

The retrospective cohort study by a team of researchers in China included 58 cataract eyes of 54 patients with corneal astigmatism who underwent phacoemulsification and rotationally asymmetric multifocal intraocular lens implantation. Patients were treated with either an opposite clear corneal incision or a single clear corneal incision.


The ASRS annual meeting will take place in Stockholm, Sweden on July 16-20, 2024. Several major updates are already anticipated at the event.
Prevent Blindness is offering educational resources and will partner with OCuSOFT Inc. in support of Dry Eye Awareness Month in July.

The award, supported by Oculis, will recognize outstanding contributions to retina research and will grant €30,000 for research support, and an additional €5,000 directly to the candidate.

Presenters from around the world converge on the Pacific Northwest to highlight innovation.

4D-175 will be evaluated in the GAZE clinical trial, an upcoming phase 1, open-label trial.

The patient-facing brochure serves as a vehicle to help ECPs review the solution’s information and prescription filling process with patients during and after an office visit, and the website serves as an additional resource and reference once the patient returns home.
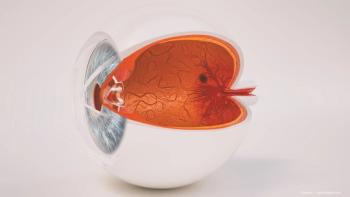
Durable treatments impact the quality of life of patients with retinal diseases.

The adaptor was tested by low-vision patients and participants with simulated visual impairment.
Physicians wonder whether normal examination results are actually normal.
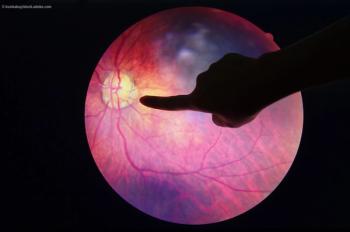
The multi-institutional found that a genetic variation common in people of African ancestry is linked with an increased risk of complications from diabetes, including diabetic retinopathy, which has previously been linked to genetic variations called single nucleotide polymorphisms, or SNPs, but these associations have been studied primarily in individuals of European and Asian ancestry.

Enhanced monofocal technology may benefit patients with cataracts and dry eye.
According to the company, its combined vitreoretinal-cataract system and standalone cataract system are cleared for use in the United States.

The results were presented at a symposium during the American Diabetes Association's 84th Scientific Sessions in Orlando, Florida, and mark the first large-scale trial specifically designed to investigate the effect of fenofibrate on eye outcomes in people with early diabetic retinopathy.

According to the company, the acquisition includes 2 commercial assets Iluvien and Yutiq, expanding ANI’s foothold in ophthalmology.

Phase 1 multicenter study demonstrates early signs of efficacy.
Researchers at Washington University School of Medicine in St. Louis for years have worked to understand the rare condition known as retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations.

A team of Korean researchers has examined data from the Korean National Health Information Database to determine whether subjects with allergic diseases exhibited a higher incidence of primary open-angle glaucoma compared to a control group.

OCU410ST is a modifier gene therapy candidate being developed for Stargardt disease, which affects approximately 100,000 people in the United States and Europe combined.

Optimizing corneal neurotization outcomes with cryopreserved amniotic membrane.
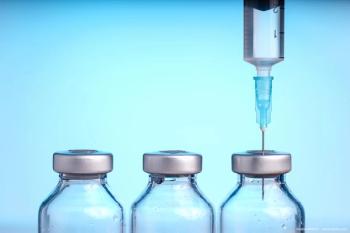
The second and third required PPQ batches are scheduled, and should they meet specifications, the new TRIESENCE production process will be complete, allowing Harrow to relaunch during 2024.

A priority review has been granted with a PDUFA goal date set for December 17, 2024.

According to the company, AURN001 is the first allogeneic cell therapy to receive both FDA designations for the treatment of corneal edema secondary to corneal endothelial disease.