
A study reveals that cryopreserved amniotic membrane enhances recovery and reduces infection risk in post-corneal cross-linking patients.

A study reveals that cryopreserved amniotic membrane enhances recovery and reduces infection risk in post-corneal cross-linking patients.

Second-year medical student Rishika Garg, from the University of Oklahoma College of Medicine in Oklahoma City, shares insights from a nationwide analysis revealing inconsistencies in ophthalmology residency leave policies.


Surgeons explore innovative cataract surgery techniques that significantly lower intraocular pressure while ensuring patient safety and comfort.

In the study, authors investigated the natural history of GA lesion incidence rates and analyzed potential risk factors for faster incidence of GA lesions.



The BIM-IOL System is for the treatment of elevated IOP in patients with mild-to-moderate open-angle glaucoma (OAG) or ocular hypertension (OHT).
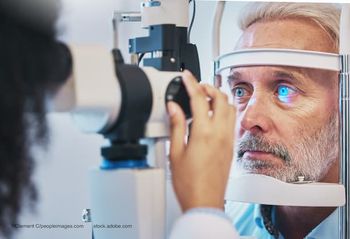
Ophthalmologists discuss how advanced imaging, MIGS, and home monitoring are reshaping decision-making in complex glaucoma cases.
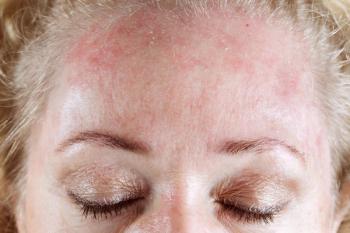
This retrospective cohort study used a large US administrative claims database to identify patients with a diagnosis of seborrheic dermatitis from January 1, 2016, through June 30, 2022.

Anat Loewenstein, MD, discusses the transformative impact of home OCT and AI on monitoring retinal diseases at AAO 2025.
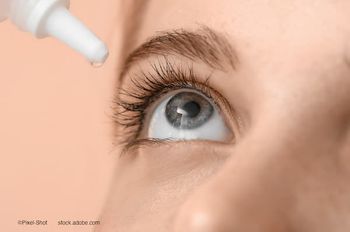
Ophthalmologists weigh in on evolving diagnostic tools, emerging therapies, and practical approaches for managing today’s most challenging ocular-surface cases.

Discover the latest advancements in laser vision correction, including next-gen LASIK techniques that achieve unprecedented visual outcomes.

Ophthalmologists weigh in on how the extended-duration therapy may reduce treatment burden for patients with wet age-related macular degeneration.

Surgeons reflect on milestones that have redefined patient care—and share a glimpse of the advances that promise to shape the next era of eye health.
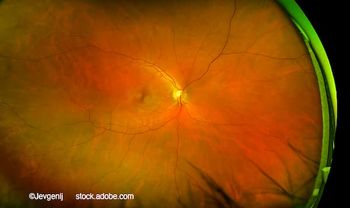
Harpal Sandhu, MD, FRCSC, discusses the preclinical performance of XPK-640 and how Optigo’s hyaluronic acid-anchoring platform may reshape dosing expectations in retinal disease.
The company describes KSI-101 as a “novel, potent, and high-strength (100 mg/mL) antibody-based investigational therapy with a bispecific mechanism of action targeting both interleukin-6 (IL-6) and vascular endothelial growth factor (VEGF).”

OPGx-LCA5 is designed to address a form of Leber congenital amaurosis that results from biallelic mutations in the LCA5 gene, which encodes the lebercilin protein, the investigators explained.

It seems there is no text provided for summarization. Please provide the text you'd like summarized, and I'll be happy to help!

PolyActiva and RareSight collaborate to create innovative therapies for rare pediatric retinal diseases, aiming to transform treatment and improve children's vision.

Tsu Shan Chambers shares how the film "My Eyes" uses authentic storytelling to raise awareness of inherited eye disease and the importance of preventive vision care.

Marking 5 decades of Ophthalmology Times, clinicians share the innovations that defined their training, shaped patient outcomes, and continue to push the field ahead.

The trial is evaluating 4D-150 in patients with wet age-related macular degeneration (wet AMD).

Host Deborah Ristvedt, DO, is joined by Marguerite B. McDonald, MD, FACS, to discuss her journey with pioneering contributions to ophthalmology, patient-centered care, leadership, and guidance for the next generation.

Johnson & Johnson reaches 100,000 TECNIS ODYSSEY IOLs implanted, and presented data on in vision correction and patient satisfaction at the AAO 2025 meeting.


The VITA Trial is a prospective pilot study on the VisiPlate Aqueous Shunt in patients with with open-angle glaucoma.
The addition comes as the company accelerates development of its long-acting anti-VEGF program and advances its HA-binding platform.

The trial met both its primary efficacy and key secondary endpoints.

The EXTEND study is a 5-year follow-up of participants who received a single intravitreal injection of MCO-010 in a previously conducted phase 1/2a trial.