
According to the company, the center, which officially opens on Thursday, September 26, will serve as a hub for hands-on training and education in lens-based vision correction.

According to the company, the center, which officially opens on Thursday, September 26, will serve as a hub for hands-on training and education in lens-based vision correction.

Moon Jeong Lee, MD, shares how volunteering in geriatric care shaped her career path.

The option gives Glaukos exclusive global license agreements on pre-agreed terms, including upfront and milestone payments plus royalties.

The study, funded by the National Institutes of Health, supports no change to surgical technique for trichiasis management.

CVD could impact physicians' ability to identify choroidal melanoma from choroidal nevus.

The company made notable hires in its biometrics, clinical operations, and market access departments.
The data were presented at the Keystone Symposium Targeting Dry Age-related Macular Degeneration: Pathophysiology and Emerging Therapies.

This Week in Ophthalmology is a video series highlighting some of the top articles featured on the Ophthalmology Times website.

Robert Osher, MD, sat down with David Hutton of Ophthalmology Times to discuss the Lifetime Achievement award he received from the European Society of Cataract and Refractive Surgeons.

Efferocytosis’ role in retinopathy is largely unknown despite knowledge of the process in other diseases.
Socioeconomic factors have long been a looming issue in various health outcomes. A team of researchers from Shanghai Jiao Tong University School of Medicine and Fudan University set out to review the impact of socioeconomic status inequality on the incidence of AMD.

American Academy of Ophthalmology offers in-person and virtual options for annual meeting, Subspecialty Day.

Oral gildeuretinol demonstrated a clinically meaningful reduction in the geographic atrophy lesion growth rate at 24 months, supporting additional clinical development. SAGA topline data has been accepted as a late breaker at the 128th annual meeting of the American Academy of Ophthalmology, being held October 18-21 in Chicago.

Cutting-edge approaches for MDs and ODs
4D Molecular Therapeutics has announced data based on the longest interim follow-up from Phase 1/2 PRISM clinical trial and 4FRONT Phase 3 study design.
According to a team of researchers at the National Institutes of Health, their findings in animals suggest a surgery-free treatment for cataracts.

Ripple will lead preclinical development of RTC-620 while AbbVie will lead the clinical and commercialization activities upon exercise of option-to-license agreement.
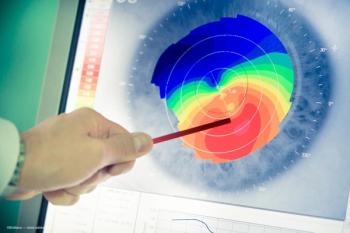
The new standard: Stabilizing keratoconus and improving vision.

IVW-1001 is a novel TRPM8 agonist delivered via ophthalmic eyelid wipe, being developed to treat DED.

Unlocking the potential of donor scleral tissue: Innovations and future applications.

The report urges the US Department of Health and Human Services, in collaboration with departments of education at the state level, to take measures to ensure that children receive a vision screening before first grade and a comprehensive eye exam, when needed.

According to the company, topline results will be presented at the 2024 American Academy of Ophthalmology Annual Meeting, being held October 18 to 21 in Chicago, Illinois.

The announcement marks the second FDA designation for ATSN-201, which previously received Rare Pediatric Disease designation.

The acquisition strengthens the company’s rare disease segment, and adds 2 durable commercial assets, Iluvien and Yutiq with significant growth potential, expanding ANI’s foothold in strategic therapeutic area of ophthalmology.

Option provides surgeons with flexibility and creativity.

German researchers found that their study results suggest that the effect of glaucoma on the elapsed time depends on disease progression. This uncertainty during everyday navigation tasks may adversely affect patients’ quality of life.
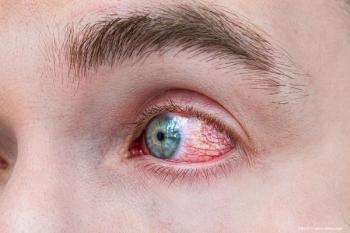
Prevent Blindness is offering free resources to help educate the public and allied healthcare professionals on the many types of inflammatory eye diseases, and steps that should be taken to prevent vision loss.

Researchers at the Karolinska Institute continue to investigate whether gene therapy can improve vision in people with Bothnia dystrophy, a form of hereditary blindness, prevalent in the region Västerbotten in Sweden.

Meeting in Stockholm provides education, updates, and a taste of culture.