Interventional Glaucoma
Latest News
Video Series

Latest Videos
Shorts
CME Content
More News

The trial will evaluate the safety and effectiveness of the company's titratable glaucoma therapy system, designed to optimize IOP reduction in patients undergoing glaucoma surgery.


James Tsai, MD, MBA, sheds light on what is in the offing for glaucoma specialists

James Tsai, MD, MBA, discusses his approaches given the wide diversity of patients in a major urban setting when awareness of glaucoma can range from highly educated patients to uninformed individuals.

Data estimates the prevalence of glaucoma currently is almost 50% higher than previously estimated and over 1.6 million individuals in the UK are estimated to have glaucoma by 2060.

The Bimatoprost Drug Pad-IOL System is intended for the lowering of intraocular pressure (IOP) in patients with open-angle glaucoma (OAG) or ocular hypertension (OHT).

“Remarkable” results were reportedly obtained with hypotony with hydroxypropyl methylcellulose.

As Glaucoma Awareness Month highlights evolving practice, specialists describe how artificial intelligence, sustained drug delivery, laser therapy, and workflow integration are reshaping earlier, more individualized glaucoma care.

Their conversation focuses on anatomy-driven, individualized approaches and multidisciplinary decision-making for patients with neovascular glaucoma.
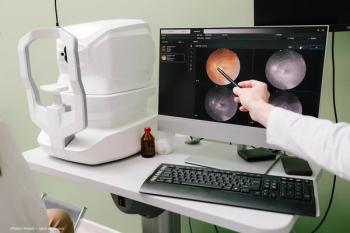
A recent study represents a step forward in investigations of the retinal layers as they are affected by glaucoma.

Joel S. Schuman, MD, FACS, highlights home tonometry, virtual visual fields, and portable OCT devices as promising tools, while noting concerns around data reliability, patient adherence, and reimbursement.

From artificial intelligence to home monitoring, Joel Schuman, MD, of Wills Eye Hospital, explores the innovations that could change how clinicians detect and treat glaucoma in the new year.


NCX 470 is Nicox’s lead dual-mechanism bimatoprost eye drop for lowering IOP in open-angle glaucoma or ocular hypertension.
The study evaluated thermal dynamics associated with CW-TSCPC and TLT using MicroPulse technology, such as temperature peak, exposure duration, and thermal spread in a simulated ciliary body using computer modeling.

The trial is evaluating the company’s femtosecond laser trabeculotomy procedure in comparison to selective laser trabeculoplasty
The SAPPHIRE trial evaluating the VisiPlate is currently underway across several sites across the US, with more site activations scheduled for 2026



The BIM-IOL System is for the treatment of elevated IOP in patients with mild-to-moderate open-angle glaucoma (OAG) or ocular hypertension (OHT).
Ophthalmologists discuss how advanced imaging, MIGS, and home monitoring are reshaping decision-making in complex glaucoma cases.

Surgeons reflect on milestones that have redefined patient care—and share a glimpse of the advances that promise to shape the next era of eye health.

The VITA Trial is a prospective pilot study on the VisiPlate Aqueous Shunt in patients with with open-angle glaucoma.
From MIGS integration to remote monitoring and next-generation therapeutics, glaucoma specialists explore how new approaches are redefining patient outcomes.

The 24-month results of this first-in-human trial supports the potential long-term outcomes of this system to lower IOP.