
At ARVO 2025, in Salt Lake City, Utah, Emma Lessieur-Contreras, MD, PhD, talked about her poster on retina extracellular vesicles, and the role that they play on diabetic retinopathy.

At ARVO 2025, in Salt Lake City, Utah, Emma Lessieur-Contreras, MD, PhD, talked about her poster on retina extracellular vesicles, and the role that they play on diabetic retinopathy.


Deep Parikh, MD, sat down with Emily Kaiser Maharjan from Optometry Times to talk about his presentation at Collaborative Care Symposium on advanced treatments in neovascular retinal disease.

At ARVO 2025, in Salt Lake City, Utah, Carly Lam, PhD, MSc, and Tsz Wing Leung, PhD talked about the evaluation of the visual performance of 2 modified DIMS spectacle lens designs.

Investigators were surprised by the outcome of a comparison between the Eyhance and enVista IOLs in low-light conditions.

The Retina World Congress showcases global innovations in surgical and medical retina, highlighting emerging treatments and fostering collaboration among specialists.

Sharon Fekrat, MD, FACS, FASRS, highlights the critical importance of networking and collaboration in the medical field, particularly in ophthalmology.

Lori Wright, JD, sat down to talk about a discussion at Collaborative Care Symposium about the risk under federal statutes and how to minimize that risk as an optometrist or an ophthalmic practice.

Early improvements in OSDI scores and tear osmolarity reinforce lifitegrast’s role in managing inflammation-driven dry eye disease.

At ARVO 2025, in Salt Lake City, Utah, Dilsher Dhoot, MD, FASRS, and Mark Barakat, MD, talked about the PHOTON trial, 8 mg versus 2 mg during the matched dosing phase, and outcomes of shortened, maintained, or extended dosing intervals

Dilraj S. Grewal, MD, FASRS, provides an in-depth overview of the current state of research and treatment innovations.

Vakharia insights from the ARCHER trial on geographic atrophy treatment for dry AMD, highlighting promising results and future directions in ophthalmology.

New analysis reveals the ellipsoidal zone's role in predicting geographic atrophy progression, enabling timely interventions to protect photoreceptors.

This partnership aims to address care gaps by identifying untreated and undertreated patients at scale.

Sunir Garg, MD, discusses a challenge in ophthalmological procedures: patient discomfort during intravitreal injections caused by povidone iodine.

Alcon’s dry eye candidate acoltremon 0.003% is a first-in-class thermoreceptor agonist, which stimulates corneal sensory nerves to increase natural tear production to treat the signs and symptoms of dry eye disease.

A conversation with David S. Friedman, MD, PhD, on treating uncontrolled open-angle glaucoma in patients with limited options

Following his keynote address at Controversies in Modern Eye Care, Dr. Maloney said he will be leaving his surgical practice and returning to research.
The investigators described the CASIA2 (Tomey Corporation) instrument, which is a new AS-OCT device with a swept-source laser wavelength of 1,310 nm that can scan at a speed of 50,000 A-scan/second.
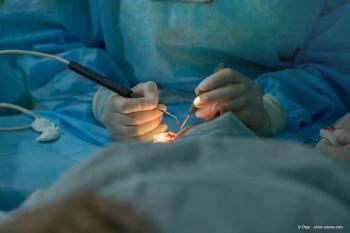
The new surgical technique incorporates a new surgical clamp that maintains eye pressure during the insertion of 2 tissue patches in immediate succession while minimizing damage to the surrounding tissue.

A significant proportion of cataract surgery patients exhibit hyperosmolar tear film both before and 1 month after surgery, underscoring preoperative screening and postoperative management to optimize visual outcomes.

ABCA4-associated retinopathies include Stargardt disease, retinitis pigmentosa 19, and cone-rod dystrophy 3.
According to the company, results showed “a meaningful clinical improvement in ocular surface health” and significance for both staining end points at day 15.

At ARVO 2025, in Salt Lake City, Utah, Steven Barnes, PhD, talked about his research on the OPA1 mutation in retinal ganglion cells' effect on the cell's electrical function.

Ayres shares insights on using a trocar-free, 27-gauge vitrector to improve surgeon comfort and patient safety in anterior vitrectomy
Swedish researchers reveal that vitamins B6, B9, B12, and choline offer neuroprotection against glaucoma, highlighting potential new treatment avenues.

At ARVO 2025, in Salt Lake City, Utah, Hsin-Yu Yang talked about the 9-month results from her study on the effectiveness of DIMS spectral lenses in myopic retardation for the pre-myopic preschoolers in Taiwan.