
According to the organization, the goal is to highlight and address the diverse and critical vision and eye health needs of children and to improve outcomes through advocacy, public health, education, and awareness.

According to the organization, the goal is to highlight and address the diverse and critical vision and eye health needs of children and to improve outcomes through advocacy, public health, education, and awareness.
Several research giants will team up to beat rare diseases that are currently using gene therapy.
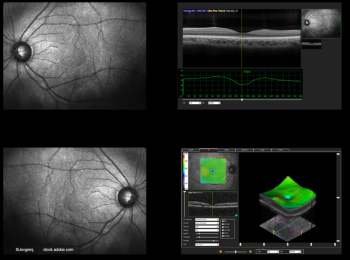
The difference in visual acuities based on CST fluctuations in these patients with diabetic macular edema remained significant.

An elevated risk may be associated with the cataract type, case complexity, and history of diabetic macular edema or proliferative diabetic retinopathy.
SD-OCT is providing reproducible, high-quality, registered images to assess the treatment response in macular disease.

According to the company, OK-101 displays both anti-inflammatory and ocular pain-reducing potential.
For surgeons, the decision goes beyond clarifying a patient’s vision.

Although graft rejection has not been associated with vaccinations, concern remains.

According to Lisa Nijm, MD, JD, patients can benefit from short-term, on-label treatment with a novel loteprednol formulation
Investigators say renal function could be used as a possible predictor for poor treatment response in certain patients with diabetic macular edema.

I-MED Pharma Inc., a Canadian-based firm, announced this week the expansion of its strategic partnership with ESW Vision in France to be their exclusive distributor in the United States.

TearLab Corp. announced that Adam Szaronos has been appointed president and CEO by the company's Board of Directors, effective immediately.
Novartis has revealed the first interpretable results from year two (week 100) of the Phase III KESTREL study.
Under terms of the agreement, ImprimisRx to assume full responsibility for US sales and marketing activities for Dexycu.

According to the company, the board will support the advancement of late-stage ophthalmic assets Nyxol and APX3330.

Investigators are hopeful that future research can focus on public health concerns of dry eye disease.

Gift marks the first time the ACMG Foundation has allocated an award for training in ophthalmic genetics.

The company’s IOPerfect combines artificial intelligence in a VR-like headset to allow tele-diagnosis and remote monitoring of glaucoma.
The company will begin commercialization of the IC-8 small aperture IOL, used for cataract patients, upon successful completion of the manufacturing facility inspections and receipt of an official approval order from the FDA, which the company estimates in Q2 2022.

The agreement includes Europe, Commonwealth of Independent States countries, China, India, parts of Latin America and the Oceania countries.

Researchers have now found that subjects who underwent cataract surgery had nearly 30% lower risk of developing dementia from any cause compared with those who did not.

Study shows statistically significant improvement for primary endpoint of bulbar conjunctival hyperemia for OTX-DED 0.2 mg and 0.3 mg formulations compared with vehicle hydrogel insert.
The approval will allow LensGen to move forward with the IDE pivotal trial with the ultimate goal of seeking premarket approval in the United States.

Omidria will become a key product in Rayner's ophthalmology franchise, which includes intraocular lenses, ophthalmic viscoelastic devices and dry eye treatments.
Save the dates for this virtual interactive conference featuring top US ophthalmologists

Successful treatment options can offer hope for patients with inherited disease.

Investigators conducted a retrospective consecutive study in which they compared the outcomes using this approach with the results achieved with local anesthesia alone during cataract surgery.

Using its proprietary software, the company has been able to create models in a fraction of the time typically needed for fitting one.

This model can restore visual function and provide good intermediate and near vision in both photopic and mesopic conditions.

According to Yvonne Ou, MD, the test can objectively detect relative changes in the on and off pathways.